Downregulation of heat shock protein 47 caused lysosomal dysfunction leading to excessive chondrocyte apoptosis
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Heat shock protein 47 (HSP47) is a collagen-specific chaperone present in several regions of the endoplasmic reticulum and cytoplasm. Elevated HSP47 expression in cells causes various cancers and fibrotic disorders. However, the consequences of HSP47 downregulation leading to chondrocyte death, as well as the underlying pathways, remain largely unclear. This study presents the first experimental evidence of the localization of HSP47 on lysosomes. Additionally, it successfully designed and generated shRNA HSP47 target sequences to suppress the expression of HSP47 in ATDC5 chondrocytes using lentiviral vectors. By employing a chondrocyte model that has undergone stable downregulation of HSP47, we observed that HSP47 downregulation in chondrocytes, disturbs the acidic homeostatic environment of chondrocyte lysosomes, causes hydrolytic enzyme activity dysregulation, impairs the lysosome-mediated autophagy-lysosome pathway, and causes abnormal expression of lysosomal morphology, number, and functional effector proteins. This implies the significance of the presence of HSP47 in maintaining proper lysosomal function. Significantly, the inhibitor CA-074 Me, which can restore the dysfunction of lysosomes, successfully reversed the negative effects of HSP47 on the autophagy-lysosomal pathway and partially reduced the occurrence of excessive cell death in chondrocytes. This suggests that maintaining proper lysosomal function is crucial for preventing HSP47-induced apoptosis in chondrocytes. The existence of HSP47 is crucial for preserving optimal lysosomal function and autophagic flux, while also inhibiting excessive apoptosis in ATDC5 chondrocytes.
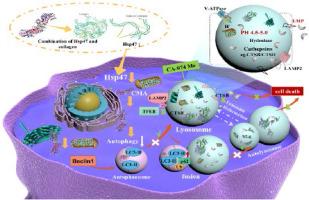
热休克蛋白 47 的下调会导致溶酶体功能障碍,从而导致软骨细胞过度凋亡。
热休克蛋白 47(HSP47)是一种胶原蛋白特异性伴侣蛋白,存在于内质网和细胞质的多个区域。细胞中 HSP47 表达升高会导致各种癌症和纤维化疾病。然而,HSP47 下调导致软骨细胞死亡的后果以及潜在的途径在很大程度上仍不清楚。本研究首次通过实验证明了 HSP47 在溶酶体上的定位。此外,该研究还成功设计并生成了 shRNA HSP47 靶序列,利用慢病毒载体抑制 HSP47 在 ATDC5 软骨细胞中的表达。通过采用稳定下调 HSP47 的软骨细胞模型,我们观察到软骨细胞中 HSP47 的下调会扰乱软骨细胞溶酶体的酸性平衡环境,导致水解酶活性失调,损害溶酶体介导的自噬-溶酶体通路,并引起溶酶体形态、数量和功能效应蛋白的异常表达。这意味着 HSP47 的存在对维持溶酶体的正常功能具有重要意义。值得注意的是,能恢复溶酶体功能障碍的抑制剂 CA-074 Me 成功逆转了 HSP47 对自噬-溶酶体通路的负面影响,并部分减少了软骨细胞过度细胞死亡的发生。这表明,维持溶酶体的正常功能对于防止 HSP47 诱导的软骨细胞凋亡至关重要。HSP47的存在对保持最佳溶酶体功能和自噬通量至关重要,同时还能抑制ATDC5软骨细胞的过度凋亡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


