Effect of isoproterenol, a β-adrenergic agonist, on the differentiation of insulin-producing pancreatic β cells derived from human pluripotent stem cells
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Research on islet replacement through the differentiation of functionally matured insulin-producing β-like cells for the treatment of diabetes presents a significant challenge. Neural signals in β cell differentiation significantly impact the pancreatic microenvironment in glucose metabolism, but they are not fully understood. In this study, isoproterenol, a β adrenoreceptor agonist, was introduced into pancreatic progenitor cells, derived from human pluripotent stem cells in vitro, undergoing endocrine differentiation, using 2-dimensional (2D) and 3-dimensional (3D) differentiation protocols. This resulted in increased insulin and C-peptide secretion, along with elevated expression of key pancreatic beta cell transcription factors, including PDX-1, NKX6.1, and MAFA, and improved function, demonstrated by increased responsiveness to glucose determined via a glucose-stimulated insulin secretion test. Moreover, RNA transcriptome analysis of isoproterenol-treated endocrine progenitors facilitated the identification of biological pathways and genes that contribute to mature beta cell differentiation efficiency correlated with neural signals, such as adrenoceptor beta 1, calcium/calmodulin dependent protein kinase II alpha, phospholipase C delta 4, and neurotrophic receptor tyrosine kinase 1. Among those genes, calcium/calmodulin dependent protein kinase II alpha was suggested as the most notable gene involved in the isoproterenol mechanism through inhibitor assays. This study illustrates that isoproterenol significantly enhances endocrine differentiation and underscores its effects on stem cell-derived beta cell maturation, emphasizing its therapeutic potential for the treatment of diabetes.
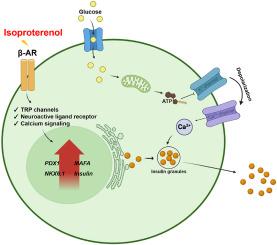
异丙肾上腺素(一种β-肾上腺素能激动剂)对人多能干细胞衍生的胰岛素分泌β细胞分化的影响。
通过分化功能成熟的胰岛素分泌β样细胞来替代胰岛治疗糖尿病的研究是一项重大挑战。β细胞分化过程中的神经信号对葡萄糖代谢过程中的胰腺微环境有重大影响,但人们对这些信号还不完全了解。在这项研究中,使用二维(2D)和三维(3D)分化方案,将异丙肾上腺素(一种β肾上腺素受体激动剂)导入体外进行内分泌分化的胰腺祖细胞(来源于人类多能干细胞)。这导致了胰岛素和C肽分泌的增加,以及关键胰腺β细胞转录因子(包括PDX-1、NKX6.1和MAFA)表达的升高,并通过葡萄糖刺激胰岛素分泌测试确定对葡萄糖反应性的增加,从而改善了功能。此外,对异丙托肾上腺素处理的内分泌祖细胞进行的 RNA 转录组分析有助于确定有助于成熟β细胞分化效率的生物通路和与神经信号相关的基因,如肾上腺素受体β1、钙/钙调蛋白依赖性蛋白激酶 II alpha、磷脂酶 C delta 4 和神经营养受体酪氨酸激酶 1。在这些基因中,钙/钙调蛋白依赖性蛋白激酶 II alpha 被认为是通过抑制剂实验参与异丙托品醇机制的最显著基因。这项研究表明,异丙托肾上腺素能显著促进内分泌分化,并强调了它对干细胞衍生的β细胞成熟的影响,强调了它治疗糖尿病的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


