Single-nucleus and spatial transcriptomics of paediatric ovary: Molecular insights into the dysregulated signalling pathways underlying premature ovarian insufficiency in classic galactosemia
Abstract
Background
Classic galactosemia (CG) is an inborn error of galactose metabolism caused by mutations in the GALT gene. Premature ovarian insufficiency (POI) is a later complication that affects 80% of women with CG due to a significant decline in ovarian reserve (primordial follicle pool). The definite mechanisms underlying the early onset of POI in CG patients are not fully understood.
Methods
In this study, we performed single-nucleus RNA sequencing (snRNA-seq) and spatial transcriptomics on ovary tissue biopsies from prepubertal girls diagnosed with CG to investigate dynamic changes in gene expression and altered signalling pathways in granulosa cells, oocytes, and stromal cells.
Results
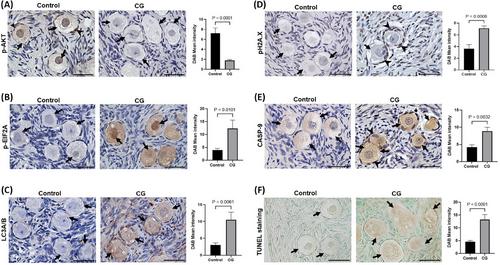
We generated single-nucleus and spatial transcriptomics atlas of human ovaries from prepubertal girls diagnosed with and without CG. snRNA-seq profiling of the paediatric ovary revealed a diverse ovarian microenvironment with seven distinct major cell types. Our transcriptomic analysis revealed an increase in the expression of several endoplasmic reticulum stress and oxidative stress associated genes, which can promote apoptosis of granulosa cells in CG. PTEN/PI3K/AKT signalling, which is crucial for primordial follicle activation and survival was dysregulated as supported by upregulated PTEN transcripts and a significant reduction in phospho-AKT levels in the granulosa cells and oocytes. We also found a marked increase in expression of phospho-H2A.X, LC3A/B and CASP9 in the primordial follicles of CG ovaries suggesting DNA damage, autophagy, and accelerated follicular atresia. Furthermore, we noticed genes participating in extracellular matrix organisation, integrin and gap junction signalling, essential for structural support of the ovarian stroma were profoundly altered.
Conclusions
Our findings provide molecular insights into the dysregulated cellular signalling pathways essential for primordial follicle growth and survival that can explain the etiology of POI in CG patients. This study has implications in the development of future therapeutic interventions to preserve ovarian function and promote female reproductive health.
Highlights
- Created a comprehensive single-nucleus transcriptomic atlas and spatial landscape of paediatric ovary tissue from prepubertal girls diagnosed with classic galactosemia (CG).
- Our transcriptomic analysis revealed activation of genes associated with ER-stress signalling, oxidative stress response and ATM signalling/DNA damage response as shown by significant increase in expression of p-EIF2A, p-H2A.X and LC3A/B in the primordial follicles of CG ovary.
- PTEN/PI3K/AKT signalling pathways was dysregulated evidenced by a significant reduction in phospho-AKT expression in the primordial follicles of CG ovary, suggesting impaired follicle activation and survival.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


