Kynurenine-AhR reduces T-cell infiltration and induces a delayed T-cell immune response by suppressing the STAT1-CXCL9/CXCL10 axis in tuberculosis
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
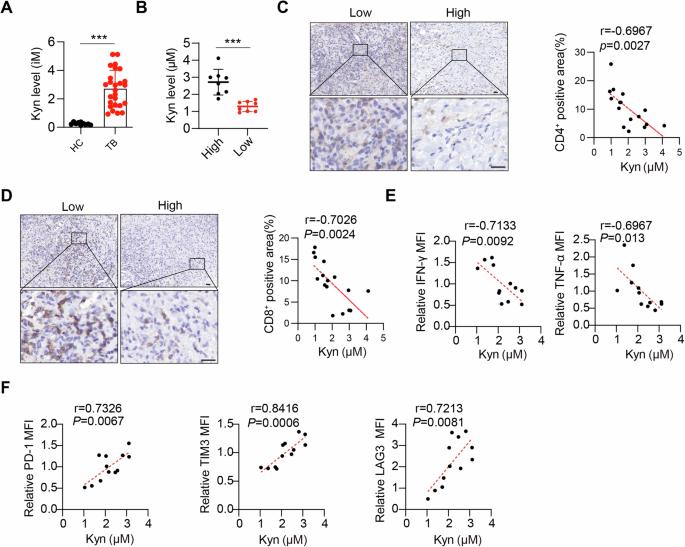
Tuberculosis, caused by Mycobacterium tuberculosis (Mtb), is a critical global health issue that is complicated by the ability of the pathogen to delay the host’s T-cell immune response. This delay in T-cell recruitment to the site of infection is a pivotal survival strategy for Mtb, allowing it to establish a persistent chronic infection. To investigate the underlying mechanisms, this study focused on Mtb’s exploitation of host tryptophan metabolism. Mtb upregulates indoleamine 2,3-dioxygenase 1 (IDO1) in inflammatory macrophages, thereby increasing kynurenine (Kyn) production. Kyn then activates the aryl hydrocarbon receptor (AhR), leading to the upregulation of suppressor of cytokine signaling 3 and subsequent inhibition of the JAK-STAT1 signaling pathway. This results in reduced secretion of the chemokines CXCL9 and CXCL10, which are crucial for T-cell recruitment to the lungs. Supported by in vivo mouse models, our findings reveal that disrupting this pathway through AhR knockout significantly enhances T-cell infiltration and activity, thereby undermining Mtb-induced immunosuppression. In contrast, additional Kyn injection obviously inhibited T-cell infiltration and activity. These results highlight potential therapeutic targets of AhR and IDO1, offering new avenues for enhancing the host immune response against tuberculosis and guiding future vaccine development efforts.
犬尿氨酸-AhR 通过抑制 STAT1-CXCL9/CXCL10 轴,减少结核病中的 T 细胞浸润并诱导延迟的 T 细胞免疫反应。
由结核分枝杆菌(Mtb)引起的结核病是一个重要的全球健康问题,由于病原体能够延迟宿主的 T 细胞免疫反应,这一问题变得更加复杂。T细胞招募到感染部位的延迟是Mtb的关键生存策略,使其能够建立持续的慢性感染。为了研究其潜在机制,本研究重点关注Mtb对宿主色氨酸代谢的利用。Mtb会上调炎症巨噬细胞中的吲哚胺2,3-二氧化酶1(IDO1),从而增加犬尿氨酸(Kyn)的产生。然后,Kyn 会激活芳基烃受体(AhR),导致细胞因子信号转导抑制因子 3 上调,进而抑制 JAK-STAT1 信号转导途径。这导致趋化因子 CXCL9 和 CXCL10 的分泌减少,而这两种趋化因子对 T 细胞招募到肺部至关重要。在体内小鼠模型的支持下,我们的研究结果表明,通过敲除 AhR 来破坏这一通路可显著增强 T 细胞的浸润和活性,从而破坏 Mtb 诱导的免疫抑制。相反,额外注射 Kyn 则明显抑制 T 细胞的浸润和活性。这些结果凸显了 AhR 和 IDO1 的潜在治疗靶点,为增强宿主对结核病的免疫反应提供了新途径,并为未来的疫苗开发工作提供了指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


