Phase II Study of Irinotecan, Trifluridine/tipiracil (TAS-102) plus Bevacizumab as a Later-line Therapy for Patients with Metastatic Colorectal Cancer (mCRC): a prospective single-center explorative study
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
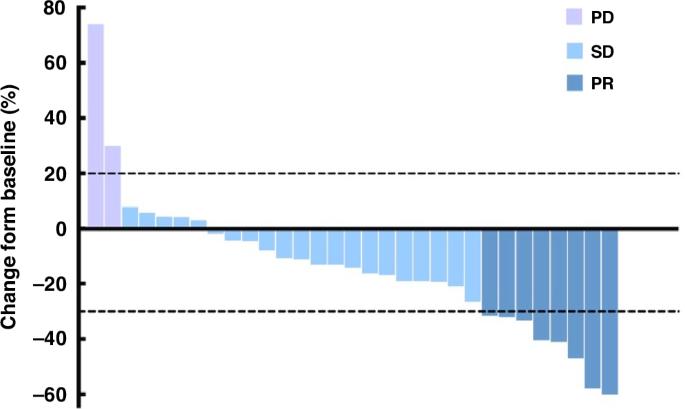
To explore the efficacy and safety of the combination of irinotecan, trifluridine/tipiracil (TAS-102), and bevacizumab in a later‐line setting for metastatic colorectal cancer (mCRC) patients. This was a single-center, phase II trial. The mCRC patients who are refractory to standard first-line and second-line treatment are eligible. Patients who previously received irinotecan while progressing during maintenance therapy are also eligible. The primary endpoint was the objective response rate (ORR). Between August 1, 2022, and September 30, 2023, 35 patients were enrolled, and 31 of them were evaluable for efficacy. The ORR was 25.8% (8/31), and the disease control rate (DCR) was 93.5% (29/31). As of April 30, 2024, the median progression-free survival (PFS) was 9.2 months (95% CI 6.285-12.115), whereas the median overall survival (OS) was not reached with the 1-year OS rate of 73.5%. The most common grade 3/4 treatment-related adverse events were neutropenia (34.3%), anemia (17.1%), and thrombocytopenia (8.6%). Irinotecan, TAS-102 plus bevacizumab regimen preliminarily demonstrated promising efficacy with tolerable toxicity for mCRC patients as later‐line treatment. This regimen warrants further exploration in refractory mCRC patients.
伊立替康、三氟啶/替比拉西(TAS-102)联合贝伐单抗作为转移性结直肠癌(mCRC)患者后期治疗的 II 期研究:一项前瞻性单中心探索性研究。
目的:探讨伊立替康、三氟啶/替比拉西(TAS-102)和贝伐珠单抗联合疗法在晚期转移性结直肠癌(mCRC)患者中的疗效和安全性:这是一项单中心 II 期试验。对标准一线和二线治疗难治的 mCRC 患者符合条件。既往接受过伊立替康治疗但在维持治疗期间病情进展的患者也符合条件。主要终点是客观反应率(ORR):2022年8月1日至2023年9月30日期间,共有35名患者入组,其中31名患者的疗效可接受评估。ORR为25.8%(8/31),疾病控制率(DCR)为93.5%(29/31)。截至2024年4月30日,中位无进展生存期(PFS)为9.2个月(95% CI 6.285-12.115),而中位总生存期(OS)尚未达到,1年OS率为73.5%。最常见的3/4级治疗相关不良事件是中性粒细胞减少(34.3%)、贫血(17.1%)和血小板减少(8.6%):结论:伊立替康、TAS-102加贝伐单抗方案作为mCRC患者的晚期治疗方案,初步显示了良好的疗效和可耐受的毒性。该方案值得在难治性mCRC患者中进一步探索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


