Bidirectional interaction between IL and 17A/IL-17RA pathway dysregulation and α-synuclein in the pathogenesis of Parkinson’s disease
IF 8.8
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Parkinson’s disease (PD) pathogenesis is characterized by α-synuclein (α-syn) pathology, which is influenced by various factors such as neuroinflammation and senescence. Increasing evidence has suggested a pivotal role for Interleukin-17A(IL-17A) and Interleukin-17 Receptor A (IL-17RA) in PD, yet the trigger and impact of IL-17A/IL-17RA activation in PD remains elusive. This study observed an age-related increase in IL-17A and IL-17RA in the human central nervous system, accompanied by increased α-syn and senescence biomarkers. Interestingly, both levels of IL-17A and IL-17RA in PD patients were significantly elevated compared to age-matched controls, wherein the IL-17A was mainly present in neurons. This abnormal neuronal IL-17A activation in the PD brain was recapitulated in α-syn mouse models. Correspondingly, administration of recombinant IL-17A exacerbated pathological α-syn in both neuron and mouse models. Furthermore, IL-17A/IL-17RA pathway interventions via blocking antibody or shRNA-mediated knockdown can mitigate the effects of pathological α-syn. This study reveals an interplay between dysregulation of the IL-17A/IL-17RA pathway and α-syn, suggesting that regulating the IL-17A/IL-17RA pathway could modify PD progression by disrupting the detrimental cycle.
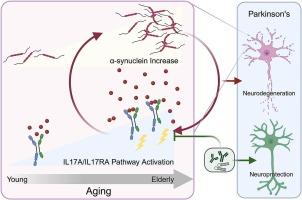
帕金森病发病机制中 IL 和 17A/IL-17RA 通路失调与 α-突触核蛋白之间的双向相互作用
帕金森病(PD)的发病机制以α-突触核蛋白(α-syn)病理学为特征,而α-突触核蛋白病理学又受到神经炎症和衰老等多种因素的影响。越来越多的证据表明,白细胞介素-17A(IL-17A)和白细胞介素-17受体A(IL-17RA)在帕金森病中起着关键作用,但IL-17A/IL-17RA激活在帕金森病中的诱因和影响仍未确定。本研究观察到人类中枢神经系统中 IL-17A 和 IL-17RA 的增加与年龄有关,同时伴随着 α-syn 和衰老生物标志物的增加。有趣的是,与年龄匹配的对照组相比,帕金森病患者体内的IL-17A和IL-17RA水平均显著升高,其中IL-17A主要存在于神经元中。在α-syn小鼠模型中,帕金森病大脑神经元IL-17A的这种异常激活也得到了重现。相应地,服用重组 IL-17A 会加剧神经元和小鼠模型中的病理 α-syn。此外,通过阻断抗体或 shRNA 介导的基因敲除干预 IL-17A/IL-17RA 通路可以减轻病理性 α-syn 的影响。这项研究揭示了IL-17A/IL-17RA通路失调与α-syn之间的相互作用,表明调节IL-17A/IL-17RA通路可以通过破坏有害循环来改变帕金森病的进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


