Pharmacological inhibition of cathepsin S and of NSPs-AAP-1 (a novel, alternative protease driving the activation of neutrophil serine proteases)
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
An uncontrolled activity of neutrophil serine proteases (NSPs) contributes to inflammatory diseases. Cathepsin C (CatC) is known to activate NSPs during neutrophilic differentiation and represents a promising pharmacological target in NSP-mediated diseases. In humans, Papillon-Lefèvre syndrome (PLS) patients have mutations in their CTSC gene, resulting in the complete absence of CatC activity. Despite this, low residual NSP activities are detected in PLS neutrophils (<10% vs healthy individuals), suggesting the involvement of CatC-independent proteolytic pathway(s) in the activation of proNSPs. This prompted us to characterize CatC-independent NSP activation pathways by blocking proCatC maturation. In this study, we show that inhibition of intracellular CatS almost completely blocked CatC maturation in human promyeloid HL-60 cells. Despite this, NSP activation was not significantly reduced, confirming the presence of a CatC-independent activation pathway involving a CatC-like protease that we termed NSPs-AAP-1. Similarly, when human CD34+ progenitor cells were treated with CatS inhibitors during neutrophilic differentiation in vitro, CatC activity was nearly abrogated but ∼30% NSP activities remained, further supporting the existence of NSPs-AAP-1. Our data indicate that NSPs-AAP-1 is a cysteine protease that is inhibited by reversible nitrile compounds designed for CatC inhibition. We further established a proof of concept for the indirect, although incomplete, inhibition of NSPs by pharmacological targeting of CatC maturation using CatS inhibitors. This emphasizes the potential of CatS as a therapeutic target for inflammatory diseases. Thus, preventing proNSP maturation using a CatS inhibitor, alone or in combination with a CatC/NSPs-AAP-1 inhibitor, represents a promising approach to efficiently control the extent of tissue injury in neutrophil-mediated inflammatory diseases.
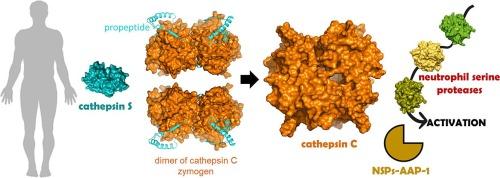
药理抑制 cathepsin S 和 NSPs-AAP-1(一种新型替代蛋白酶,可驱动中性粒细胞丝氨酸蛋白酶的活化)。
中性粒细胞丝氨酸蛋白酶(NSPs)的活性失控是导致炎症性疾病的原因之一。众所周知,Cathepsin C(CatC)能在中性粒细胞分化过程中激活NSP,是治疗NSP介导的疾病的药物靶点。在人类中,巴比隆-勒菲弗综合征(PLS)患者的CTSC基因发生突变,导致CatC活性完全缺失。尽管如此,在 PLS 嗜中性粒细胞中仍能检测到较低的残余 NSP 活性(在体外嗜中性粒细胞分化过程中用 CatS 抑制剂处理 + 祖细胞,CatC 活性几乎消失,但仍有 30% 的 NSP 活性,这进一步证明了 NSPs-AAP-1 的存在)。我们的数据表明,NSPs-AAP-1 是一种半胱氨酸蛋白酶,可被设计用于抑制 CatC 的可逆腈化合物抑制。我们进一步证明了利用 CatS 抑制剂以 CatC 成熟为药理靶点间接抑制 NSPs 的概念,尽管这种抑制并不完全。这强调了 CatS 作为炎症性疾病治疗靶点的潜力。因此,使用 CatS 抑制剂单独或与 CatC/NSPs-AAP-1 抑制剂联合防止原 NSP 成熟是有效控制中性粒细胞介导的炎症性疾病中组织损伤程度的一种很有前景的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


