Unraveling the Membrane Topology of TMEM151A: A Step Towards Understanding its Cellular Role
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Transmembrane protein 151A (TMEM151A) has been identified as a causative gene for paroxysmal kinesigenic dyskinesia, though its molecular function remains almost completely unknown. Understanding the membrane topology of transmembrane proteins is crucial for elucidating their functions and possible interacting partners. In this study, we utilized molecular dynamics simulations, immunocytochemistry, and electron microscopy to define the topology of TMEM151A. Our results validate a starting AlphaFold model of TMEM151A and reveal that it comprises a transmembrane domain with two membrane-spanning alpha helices connected by a short extracellular loop and an intramembrane helix-hinge-helix structure. Notably, most of the protein is oriented towards the intracellular side of the membranes with a large cytosolic domain featuring a combination of alpha-helix and beta-sheet structures, as well as the protein N- and C-termini. These insights into TMEM151A’s topology and orientation of its domains with respect of the cell membranes provide essential information for future functional studies and represent a first fundamental step for understanding its role in the pathogenesis of paroxysmal kinesigenic dyskinesia.
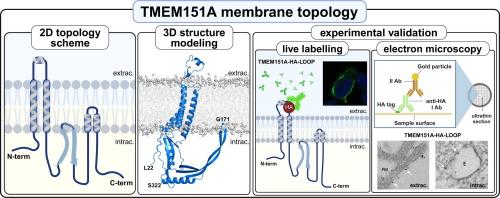
揭示 TMEM151A 的膜拓扑结构:了解其细胞作用的一步。
跨膜蛋白 151A(TMEM151A)已被确定为阵发性运动障碍的致病基因,但其分子功能几乎完全未知。了解跨膜蛋白的膜拓扑结构对于阐明其功能和可能的相互作用伙伴至关重要。在本研究中,我们利用分子动力学模拟、免疫细胞化学和电子显微镜确定了 TMEM151A 的拓扑结构。我们的研究结果验证了 TMEM151A 的起始 AlphaFold 模型,并揭示了它由一个跨膜结构域和两个跨膜 alpha 螺旋组成,两个跨膜 alpha 螺旋由一个短的胞外环和一个膜内螺旋-铰链-螺旋结构连接。值得注意的是,该蛋白质的大部分都面向膜的细胞内侧,其中一个大的细胞膜结构域具有α-螺旋和β-片状结构的组合,以及蛋白质的 N 端和 C 端。对 TMEM151A 的拓扑结构及其结构域在细胞膜上的取向的深入研究为今后的功能研究提供了重要信息,也为了解其在阵发性运动障碍发病机制中的作用迈出了基础性的第一步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


