Disruption of CAD Oligomerization by Pathogenic Variants
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
CAD, the multi-enzymatic protein essential for initiating the de novo biosynthesis of pyrimidine nucleotides, forms large hexamers whose structure and function are not fully understood. Defects in CAD cause a severe neurometabolic disorder that is challenging to diagnose. We developed a cellular functional assay to identify defective CAD variants, and in this study, we characterized five pathogenic missense mutations in CAD’s dihydroorotase (DHO) and aspartate transcarbamoylase (ATC) domains. All mutations impaired enzymatic activities, with two notably disrupting the formation of DHO dimers and ATC trimers. Combining crystal structures and AlphaFold predictions, we modeled the hexameric CAD complex, highlighting the central role of the DHO and ATC domains in its assembly. Our findings provide insight into CAD’s stability, function, and organization, revealing that correct oligomerization of CAD into a supramolecular complex is required for its function in nucleotide synthesis and that mutations affecting this assembly are potentially pathogenic.
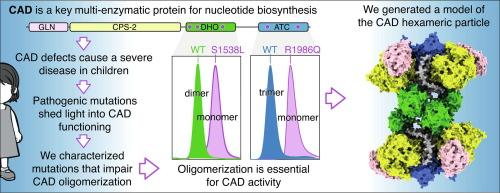
致病变体对 CAD 寡聚化的破坏。
CAD是启动嘧啶核苷酸从头生物合成所必需的多酶蛋白,可形成大型六聚体,其结构和功能尚不完全清楚。CAD缺陷会导致严重的神经代谢紊乱,而这种紊乱的诊断具有挑战性。我们开发了一种细胞功能检测方法来鉴定有缺陷的 CAD 变异体,在这项研究中,我们鉴定了 CAD 的二氢烟酸酶(DHO)和天冬氨酸转氨酶(ATC)结构域中的五个致病性错义突变。所有突变都损害了酶活性,其中两个突变明显破坏了 DHO 二聚体和 ATC 三聚体的形成。结合晶体结构和 AlphaFold 预测,我们对六聚体 CAD 复合物进行了建模,突出了 DHO 和 ATC 结构域在其组装中的核心作用。我们的研究结果提供了对 CAD 的稳定性、功能和组织的深入了解,揭示了 CAD 正确寡聚成超分子复合物是其发挥核苷酸合成功能的必要条件,而影响这种组装的突变具有潜在的致病性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


