Catalytic and post-translational maturation roles of a conserved active site serine residue in nitrile hydratases
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
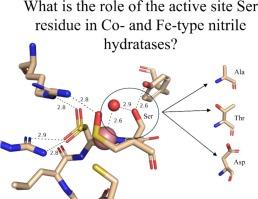
A highly conserved second-sphere active site αSer residue in nitrile hydratase (NHase), that forms a hydrogen bond with the axial metal-bound water molecule, was mutated to Ala, Asp, and Thr, in the Co-type NHase from Pseudonocardia thermophila JCM 3095 (PtNHase) and to Ala and Thr in the Fe-type NHase from Rhodococcus equi TG328–2 (ReNHase). All five mutants were successfully purified; metal analysis via ICP-AES indicated that all three Co-type PtNHase mutants were in their apo-form while the Fe-type αSer117Ala and αSer117Thr mutants contained 85 and 50 % of their active site Fe(III) ions, respectively. The kcat values obtained for the PtNHase mutant enzymes were between 0.03 ± 0.01 and 0.2 ± 0.02 s−1 amounting to <0.8 % of the kcat value observed for WT PtNHase. The Fe-type ReNHase mutants retained some detectable activity with kcat values of 93 ± 3 and 40 ± 2 s−1 for the αSer117Ala and αSer117Thr mutants, respectively, which is ∼5 % of WT ReNHase activity towards acrylonitrile. UV–Vis spectra coupled with EPR data obtained on the ReNHase mutant enzymes showed subtle changes in the electronic environment around the active site Fe(III) ions, consistent with altering the hydrogen bonding interaction with the axial water ligand. X-ray crystal structures of the three PtNHase mutant enzymes confirmed the mutation and the lack of active site metal, while also providing insight into the active site hydrogen bonding network. Taken together, these data confirm that the conserved active site αSer residue plays an important catalytic role but is not essential for catalysis. They also confirm the necessity of the conserved second-sphere αSer residue for the metalation process and subsequent post-translational modification of the α-subunit in Co-type NHases but not Fe-type NHases, suggesting different mechanisms for the two types of NHases.
Synopsis
A strictly conserved active site αSer residue in both Co- and Fe-type nitrile hydratases was mutated. This αSer residue was found to play an important catalytic function, but is not essential. In Co-type NHases, it appears to be essential for active site maturation, but not in Fe-type NHases.
腈水解酶中一个保守的活性位点丝氨酸残基的催化和翻译后成熟作用。
腈水解酶(NHase)中与轴向金属结合水分子形成氢键的高度保守的第二球活性位点αSer残基在嗜热假心杆菌JCM 3095的共型NHase(PtNHase)中被突变为Ala、Asp和Thr,在等球红球菌TG328-2的铁型NHase(ReNHase)中被突变为Ala和Thr。通过 ICP-AES 进行的金属分析表明,所有三种 Co 型 PtNHase 突变体均为其apo-form,而 Fe 型 αSer117Ala 和 αSer117Thr 突变体分别含有 85% 和 50% 的活性位点 Fe(III) 离子。PtNHase 突变体酶的 kcat 值介于 0.03 ± 0.01 和 0.2 ± 0.02 s-1 之间,与 WT PtNHase 的 cat 值相当。Fe 型 ReNHase 突变体保留了一些可检测到的活性,αSer117Ala 和 αSer117Thr 突变体的 kcat 值分别为 93 ± 3 和 40 ± 2 s-1,相当于 WT ReNHase 对丙烯腈活性的 5%。在 ReNHase 突变体酶上获得的紫外-可见光谱和 EPR 数据显示,活性位点铁(III)离子周围的电子环境发生了微妙的变化,这与改变与轴向水配体的氢键相互作用是一致的。三种 PtNHase 突变体酶的 X 射线晶体结构证实了突变和活性位点金属的缺乏,同时也提供了对活性位点氢键网络的深入了解。总之,这些数据证实了保守的活性位点αSer残基起着重要的催化作用,但并非催化所必需。这些数据还证实,在共价型 NHase 而非铁价型 NHase 中,保守的第二球 αSer 残基对于 α 亚基的金属化过程和随后的翻译后修饰是必需的,这表明这两种类型的 NHase 具有不同的机制。简述:Co 型和 Fe 型腈水解酶中一个严格保守的活性位点 αSer 残基发生了突变。研究发现,该 αSer 残基具有重要的催化功能,但并非必不可少。在 Co 型腈水解酶中,它似乎对活性位点的成熟至关重要,但在 Fe 型腈水解酶中并非如此。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


