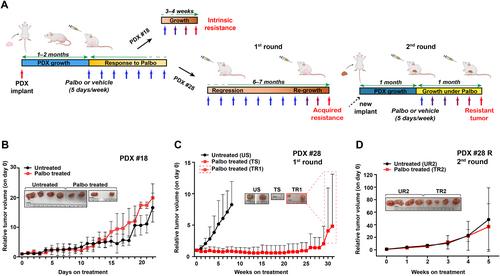
A comprehensive luminal breast cancer patient-derived xenografts (PDX) library to capture tumor heterogeneity and explore the mechanisms of resistance to CDK4/6 inhibitors
IF 5.2
2区 医学
Q1 ONCOLOGY
Ilenia Segatto, Maria Chiara Mattevi, Gian Luca Rampioni Vinciguerra, Nicole Crestan, Lorena Musco, Andrea Favero, Alessandra Dall'Acqua, Gabriele Di Giustino, Giorgia Mungo, Sara D'Andrea, Chiara Gava, Federica Ruggiero, Matteo Dugo, Lorenzo Gerratana, Fabio Puglisi, Samuele Massarut, Riccardo Bomben, Maurizio Callari, Tiziana Perin, Gustavo Baldassarre, Barbara Belletti
下载PDF
{"title":"A comprehensive luminal breast cancer patient-derived xenografts (PDX) library to capture tumor heterogeneity and explore the mechanisms of resistance to CDK4/6 inhibitors","authors":"Ilenia Segatto, Maria Chiara Mattevi, Gian Luca Rampioni Vinciguerra, Nicole Crestan, Lorena Musco, Andrea Favero, Alessandra Dall'Acqua, Gabriele Di Giustino, Giorgia Mungo, Sara D'Andrea, Chiara Gava, Federica Ruggiero, Matteo Dugo, Lorenzo Gerratana, Fabio Puglisi, Samuele Massarut, Riccardo Bomben, Maurizio Callari, Tiziana Perin, Gustavo Baldassarre, Barbara Belletti","doi":"10.1002/path.6358","DOIUrl":null,"url":null,"abstract":"<p>Breast cancer (BC) is marked by significant genetic, morphological and clinical heterogeneity. To capture this heterogeneity and unravel the molecular mechanisms driving tumor progression and drug resistance, we established a comprehensive patient-derived xenograft (PDX) biobank, focusing particularly on luminal (estrogen receptor, ER+) and young premenopausal patients, for whom PDX models are currently scarce. Across all BC subtypes, our efforts resulted in an overall success rate of 17% (26 established PDX lines out of 151 total attempts), specifically 15% in luminal, 12% in human epidermal growth factor receptor 2 positive (HER2+) and 35% in triple negative BC. These PDX mirrored morphologic and genetic features of BC from which they originated, serving as a reliable tool to investigate drug resistance and test therapeutic strategies. We focused on understanding resistance to CDK4/6 inhibitors (CDK4/6i), which are crucial in the treatment of patients with advanced luminal BC. Treating a sensitive luminal BC PDX with the CDK4/6i palbociclib revealed that, despite initial tumor shrinkage, some tumors might eventually regrow under drug treatment. RNA sequencing, followed by gene set enrichment analyses, unveiled that these PDXs have become refractory to CDK4/6i, both at biological and molecular levels, displaying significant enrichment in proliferation pathways, such as <i>MTORC1</i>, <i>E2F</i> and <i>MYC</i>. Using organoids derived from these PDX (PDxO), we observed that acquisition of CDK4/6i resistance conferred cross-resistance to endocrine therapy and that targeting MTORC1 was a successful strategy to overcome CDK4/6i resistance. Considered together, these results indicate that our PDX models may serve as robust tools to elucidate the molecular basis of BC disease progression and, by providing the possibility to simultaneously test different therapies on the same tumor, to surmount treatment resistance. While this approach is of course not feasible in the clinic, its exploitation in PDX may expedite the identification and development of more successful therapies for patients with advanced luminal BC. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 4","pages":"434-447"},"PeriodicalIF":5.2000,"publicationDate":"2024-10-25","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6358","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6358","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Breast cancer (BC) is marked by significant genetic, morphological and clinical heterogeneity. To capture this heterogeneity and unravel the molecular mechanisms driving tumor progression and drug resistance, we established a comprehensive patient-derived xenograft (PDX) biobank, focusing particularly on luminal (estrogen receptor, ER+) and young premenopausal patients, for whom PDX models are currently scarce. Across all BC subtypes, our efforts resulted in an overall success rate of 17% (26 established PDX lines out of 151 total attempts), specifically 15% in luminal, 12% in human epidermal growth factor receptor 2 positive (HER2+) and 35% in triple negative BC. These PDX mirrored morphologic and genetic features of BC from which they originated, serving as a reliable tool to investigate drug resistance and test therapeutic strategies. We focused on understanding resistance to CDK4/6 inhibitors (CDK4/6i), which are crucial in the treatment of patients with advanced luminal BC. Treating a sensitive luminal BC PDX with the CDK4/6i palbociclib revealed that, despite initial tumor shrinkage, some tumors might eventually regrow under drug treatment. RNA sequencing, followed by gene set enrichment analyses, unveiled that these PDXs have become refractory to CDK4/6i, both at biological and molecular levels, displaying significant enrichment in proliferation pathways, such as MTORC1 , E2F and MYC . Using organoids derived from these PDX (PDxO), we observed that acquisition of CDK4/6i resistance conferred cross-resistance to endocrine therapy and that targeting MTORC1 was a successful strategy to overcome CDK4/6i resistance. Considered together, these results indicate that our PDX models may serve as robust tools to elucidate the molecular basis of BC disease progression and, by providing the possibility to simultaneously test different therapies on the same tumor, to surmount treatment resistance. While this approach is of course not feasible in the clinic, its exploitation in PDX may expedite the identification and development of more successful therapies for patients with advanced luminal BC. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
用于捕捉肿瘤异质性和探索 CDK4/6 抑制剂抗药性机制的综合腔隙性乳腺癌患者衍生异种移植物 (PDX) 库。
乳腺癌(BC)具有显著的遗传、形态和临床异质性。为了捕捉这种异质性并揭示驱动肿瘤进展和耐药性的分子机制,我们建立了一个全面的患者来源异种移植物(PDX)生物库,尤其关注腔隙性(雌激素受体,ER+)和绝经前年轻患者,因为目前缺乏针对这些患者的 PDX 模型。通过我们的努力,所有 BC 亚型的总体成功率为 17%(在总共 151 次尝试中建立了 26 个 PDX 株系),其中管腔型为 15%,人表皮生长因子受体 2 阳性(HER2+)为 12%,三阴性 BC 为 35%。这些 PDX 反映了 BC 的形态学和遗传学特征,是研究耐药性和测试治疗策略的可靠工具。我们重点了解了CDK4/6抑制剂(CDK4/6i)的耐药性,CDK4/6i是治疗晚期管腔型BC患者的关键。用CDK4/6i帕博西尼(palbociclib)治疗敏感的管腔型BC PDX发现,尽管最初肿瘤缩小,但一些肿瘤最终可能会在药物治疗下重新生长。RNA测序以及随后的基因组富集分析揭示,这些PDX在生物学和分子水平上都对CDK4/6i产生了耐药性,在MTORC1、E2F和MYC等增殖通路中显示出显著的富集。我们利用从这些PDX(PDxO)中提取的器官组织观察到,CDK4/6i耐药会导致对内分泌治疗的交叉耐药,而靶向MTORC1是克服CDK4/6i耐药的成功策略。综合来看,这些结果表明我们的PDX模型可以作为强有力的工具,用于阐明BC疾病进展的分子基础,并通过提供在同一肿瘤上同时测试不同疗法的可能性来克服耐药性。当然,这种方法在临床上并不可行,但在 PDX 中利用这种方法可能会加快为晚期管腔癌患者确定和开发更成功的疗法。© 2024 作者。病理学杂志》由约翰威利父子有限公司代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
来源期刊
期刊介绍:
The Journal of Pathology aims to serve as a translational bridge between basic biomedical science and clinical medicine with particular emphasis on, but not restricted to, tissue based studies. The main interests of the Journal lie in publishing studies that further our understanding the pathophysiological and pathogenetic mechanisms of human disease.
The Journal of Pathology welcomes investigative studies on human tissues, in vitro and in vivo experimental studies, and investigations based on animal models with a clear relevance to human disease, including transgenic systems.
As well as original research papers, the Journal seeks to provide rapid publication in a variety of other formats, including editorials, review articles, commentaries and perspectives and other features, both contributed and solicited.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


