Structure-guided design and photochemical synthesis of new carbamo(dithioperoxo)thioates with improved potencies to SARS-CoV-2 3CLpro
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
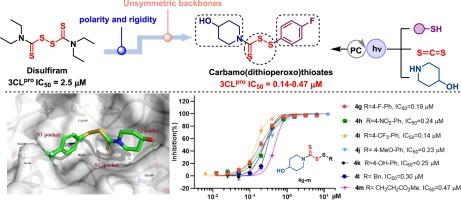
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has triggered a protracted global pandemic from 2019 to 2022, and posed a significant threat to human health. One of the non-structural proteins 3CLpro of SARS-CoV-2 is considered as a validated target for the development of inhibitors against the virus. Disulfiram has been reported as a covalent inhibitor of 3CLpro; however, its structure lacks bonding site with active pockets of 3CLpro and its highly symmetric structure doesn’t match well with the irregular cavity of the active center, limiting its therapeutic applications. To enhance their affinity for the 3CLpro target, in this study, two kinds of disulfiram derivatives, designed based on the reevaluation and optimization of disulfiram, have been synthesized through photoredox chemistry, and the novel carbamo(dithioperoxo)thioates 4g-m were found to display 5–17 folds potency against SARS-CoV-2 3CLpro compared to the parent disulfiram, with resulting half-maximal inhibitory concentration (IC50) values ranging from 0.14–0.47 μM. Carbamo(dithioperoxo)thioates 4i containing a 4-hydroxy piperidine and a 4-trifluoromethyl phenyl ring, was identified as the most potent inhibitor to both 3CLpro (IC50 = 0.14 μM) and PLpro (IC50 = 0.04 μM). Furthermore, molecular dynamics simulations, binding free energy analysis and mass analysis were performed and provided insights on the stability, conformational behavior, and interactions of 4g with 3CLpro. The green synthetic methodology, the privileged carbamo(dithioperoxo)thioate scaffold, and the molecular mechanisms presented might serve as a useful system for the further discovery of highly potent inhibitors targeting SARS-CoV-2 3CLpro.
结构引导设计和光化学合成对 SARS-CoV-2 3CLpro 有更好药效的新型氨基甲酰(二硫代丙氧)硫酸盐。
由SARS-CoV-2病毒引起的COVID-19大流行引发了2019年至2022年旷日持久的全球大流行,对人类健康构成了重大威胁。SARS-CoV-2 的非结构蛋白之一 3CLpro 被认为是开发病毒抑制剂的有效靶点。据报道,双硫仑是 3CLpro 的共价抑制剂,但其结构缺乏与 3CLpro 活性袋的结合位点,而且其高度对称的结构与活性中心的不规则空腔不太匹配,限制了其治疗应用。为了提高其对3CLpro靶点的亲和力,本研究在对双硫仑进行重新评估和优化的基础上,通过光氧化化学反应合成了两种双硫仑衍生物,并发现新型硫代氨甲酰氨基甲烷4g-m与母体双硫仑相比,对SARS-CoV-2 3CLpro的效力提高了5-17倍,其半最大抑制浓度(IC50)值在0.14-0.47 μM之间。含有一个 4-羟基哌啶和一个 4-三氟甲基苯基环的硫代氨基甲酸碳酰胺 4i 被确定为对 3CLpro (IC50 = 0.14 μM)和 PLpro (IC50 = 0.04 μM)最有效的抑制剂。此外,还进行了分子动力学模拟、结合自由能分析和质量分析,并对 4g 的稳定性、构象行为以及与 3CLpro 的相互作用进行了深入研究。所提出的绿色合成方法、特殊的氨基甲酰(二硫代丙氧)硫酸盐支架和分子机制可作为进一步发现针对 SARS-CoV-2 3CLpro 的高效抑制剂的有用系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


