Catalytic Activation of Thioglycosides with Copper-Carbenes for Stereoselective 1,2-Cis-Furanosylations.
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Thioglycoside activation, crucial for oligosaccharide synthesis, faces challenges with the need for stoichiometric promoters, additives, and cryogenic conditions, particularly in stereoselective 1,2-cis-linkage formation. This study introduces a carbene-based catalytic method using Cu(OTf)2 for thioglycoside activation, enabling efficient 1,2-cis-furanosylation in ribose and arabinose. The method is orthogonal to conventional thioglycoside and alkyne donors, accommodates sterically demanding acceptors, and achieves stereoselectivity independent of the donor anomeric configuration and C2-protecting groups, with copper chelation playing a key role.
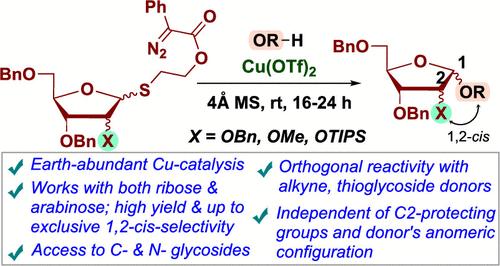
用碳酸铜催化活化硫代糖苷,实现立体选择性 1,2-Cis-Furanosylations.
硫代糖苷的活化是寡糖合成的关键,但它面临着各种挑战,需要符合一定比例的促进剂、添加剂和低温条件,特别是在立体选择性 1,2-顺式连接形成方面。本研究介绍了一种基于碳的催化方法,利用 Cu(OTf)2 活化硫代糖苷,从而在核糖和阿拉伯糖中实现高效的 1,2-顺式呋喃糖基化。该方法与传统的硫代糖苷和炔烃供体正交,可容纳立体要求较高的受体,并在铜螯合的关键作用下,实现与供体异构体构型和 C2 保护基团无关的立体选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


