RIP1 inhibition protects retinal ganglion cells in glaucoma models of ocular injury
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Receptor-interacting protein 1 (RIP1, RIPK1) is a critical mediator of multiple signaling pathways that promote inflammatory responses and cell death. The kinase activity of RIP1 contributes to the pathogenesis of a number of inflammatory and neurodegenerative diseases. However, the role of RIP1 in retinopathies remains unclear. This study demonstrates that RIP1 inhibition protects retinal ganglion cells (RGCs) in preclinical glaucoma models. Genetic inactivation of RIP1 improves RGC survival and preserves retinal function in the preclinical glaucoma models of optic nerve crush (ONC) and ischemia–reperfusion injury (IRI). In addition, the involvement of necroptosis in ONC and IRI glaucoma models was examined by utilizing RIP1 kinase-dead (RIP1-KD), RIP3 knockout (RIP3-KO), and MLKL knockout (MLKL-KO) mice. The number of RGCs, retinal thickness, and visual acuity were rescued in RIP1-kinase-dead (RIP1-KD) mice in both models, while wild-type (WT) mice experienced significant retinal thinning, RGC loss, and vision impairment. RIP3-KO and MLKL-KO mice showed moderate protective effects in the IRI model and limited in the ONC model. Furthermore, we confirmed that a glaucoma causative mutation in optineurin, OPTN-E50K, sensitizes cells to RIP1-mediated inflammatory cell death. RIP1 inhibition reduces RGC death and axonal degeneration following IRI in mice expressing OPTN-WT and OPTN-E50K variant mice. We demonstrate that RIP1 inactivation suppressed microglial infiltration in the RGC layer following glaucomatous damage. Finally, this study highlights that human glaucomatous retinas exhibit elevated levels of TNF and RIP3 mRNA and microglia infiltration, thus demonstrating the role of neuroinflammation in glaucoma pathogenesis. Altogether, these data indicate that RIP1 plays an important role in modulating neuroinflammation and that inhibiting RIP1 activity may provide a neuroprotective therapy for glaucoma.

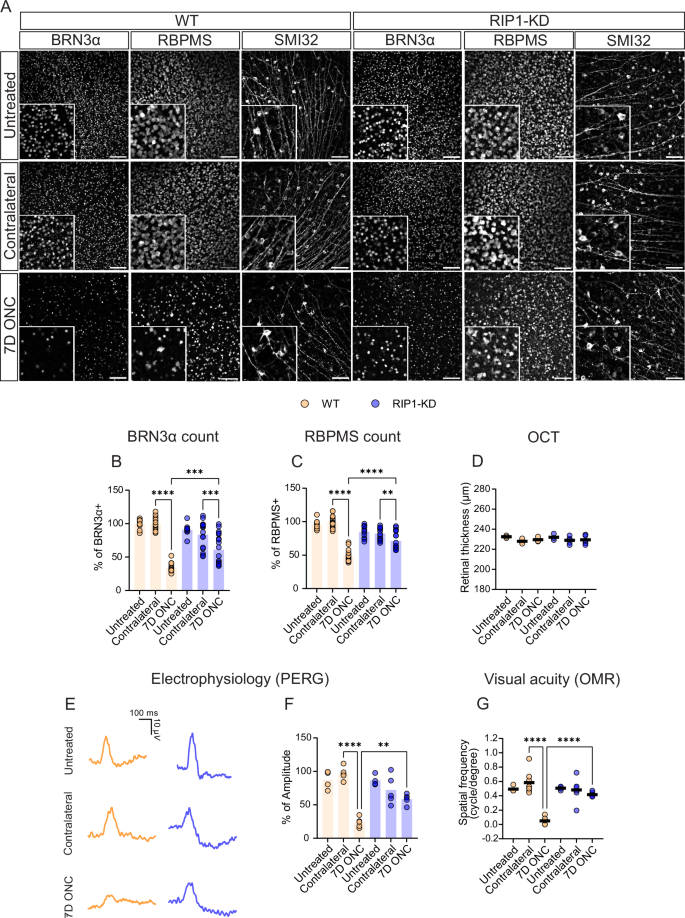
抑制 RIP1 可保护青光眼眼损伤模型中的视网膜神经节细胞
受体相互作用蛋白 1(RIP1,RIPK1)是促进炎症反应和细胞死亡的多种信号通路的关键介质。RIP1 的激酶活性是多种炎症和神经退行性疾病的发病机制之一。然而,RIP1 在视网膜病变中的作用仍不清楚。本研究证明,在临床前青光眼模型中,抑制 RIP1 可保护视网膜神经节细胞(RGC)。在视神经压迫(ONC)和缺血再灌注损伤(IRI)的临床前青光眼模型中,RIP1基因失活可提高RGC的存活率并保护视网膜功能。此外,还利用RIP1激酶凋亡(RIP1-KD)、RIP3基因敲除(RIP3-KO)和MLKL基因敲除(MLKL-KO)小鼠,研究了坏死凋亡在ONC和IRI青光眼模型中的参与情况。在这两种模型中,RIP1 激酶致死(RIP1-KD)小鼠的 RGC 数量、视网膜厚度和视敏度都得到了挽救,而野生型(WT)小鼠则出现了明显的视网膜变薄、RGC 丢失和视力损伤。RIP3-KO和MLKL-KO小鼠在IRI模型中表现出适度的保护作用,而在ONC模型中则表现出有限的保护作用。此外,我们还证实,青光眼的致病突变视神经蛋白(OPTN-E50K)会使细胞对 RIP1 介导的炎性细胞死亡敏感。在表达 OPTN-WT 和 OPTN-E50K 变异小鼠中,抑制 RIP1 可减少 IRI 后 RGC 的死亡和轴突变性。我们证明,RIP1 失活抑制了青光眼损伤后 RGC 层的小胶质细胞浸润。最后,本研究强调了人类青光眼视网膜表现出 TNF 和 RIP3 mRNA 水平升高以及小胶质细胞浸润,从而证明了神经炎症在青光眼发病机制中的作用。总之,这些数据表明,RIP1 在调节神经炎症中发挥着重要作用,抑制 RIP1 的活性可为青光眼提供神经保护疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


