Characterization of clonal dynamics using duplex sequencing in donor-recipient pairs decades after hematopoietic cell transplantation
IF 14.6
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
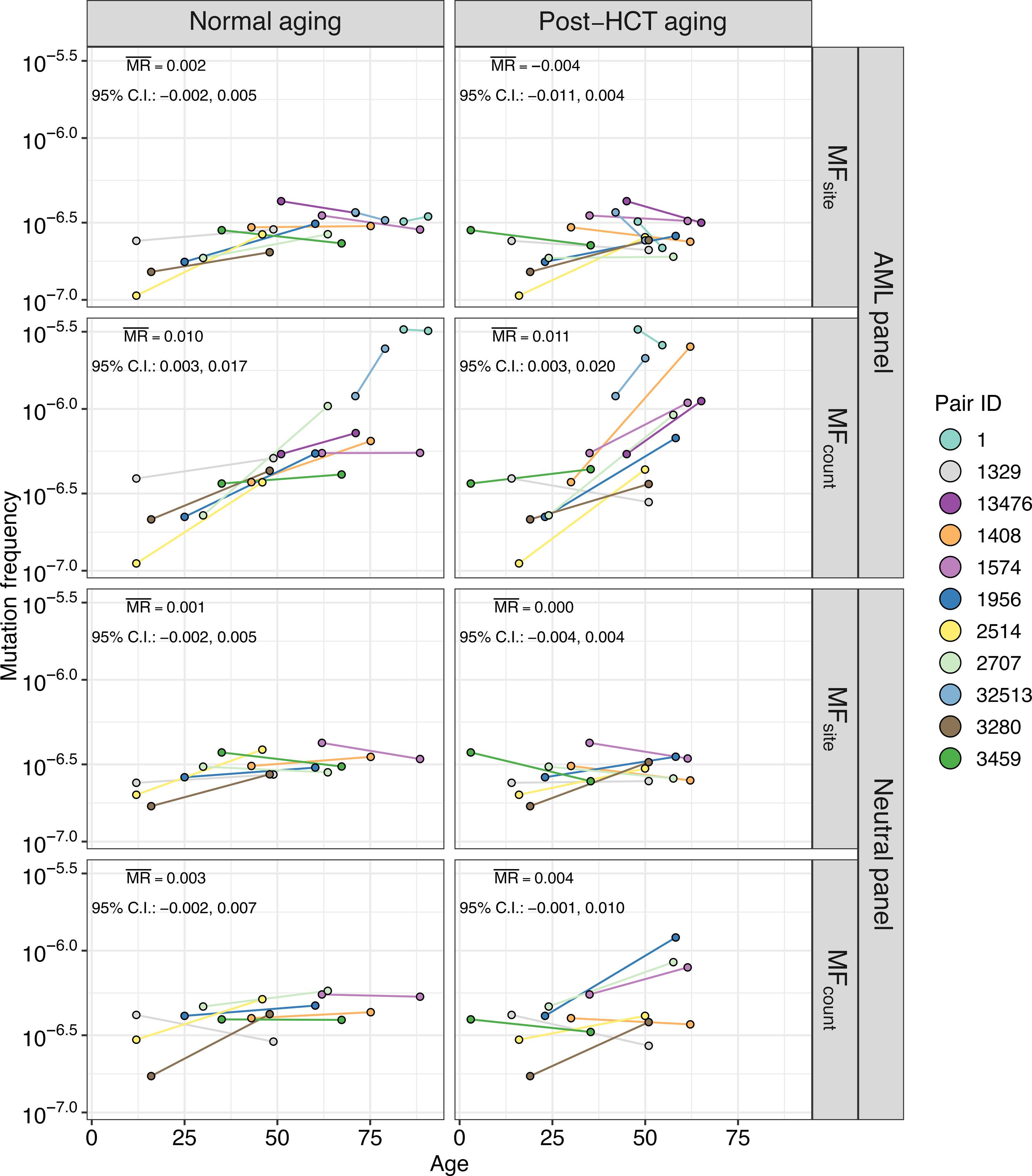
After allogeneic hematopoietic cell transplantation (HCT), a very small number of donor stem cells reconstitute the recipient hematopoietic system, whereas the donor is left with a near-normal pool of stem cells. We hypothesized that the increased replicative stress on transplanted donor cells in the recipient could lead to the disproportionate proliferation of clonal hematopoiesis (CH) variants. We obtained blood samples from 16 related donor-recipient pairs at a median of 33.8 years (range: 6.6 to 45.7) after HCT, including the longest surviving HCT recipients in the world. For 11 of 16 pairs, a donor sample from the time of HCT was available for comparison. We performed ultrasensitive duplex sequencing of genes recurrently mutated in myeloid malignancies and CH, as well as a set of functionally neutral genomic regions representative of human genomic content at large. CH variants were observed in all donors, even those as young as 12 years old. Where donor pre-HCT sample was available, the average mutation rate in donors compared to recipients post-HCT was similar (2.0% versus 2.6% per year, respectively) within genes recurrently mutated in myeloid malignancies. Twenty-two (5.6%) of the 393 variants shared between paired donors and recipients post-HCT showed ≥10-fold higher variant allele frequency (VAF) in the recipient. A longer time since HCT was positively associated with the expansion of shared variant VAFs in the recipient. In conclusion, even decades after HCT, there does not appear to be widespread accelerated clonal expansion in the transplanted cells, highlighting the immense regenerative capacity of the human hematopoietic system.
利用双链测序分析造血细胞移植数十年后捐赠者-受者配对的克隆动态特征
异基因造血细胞移植(HCT)后,极少数供体干细胞会重建受体的造血系统,而供体则只剩下一个接近正常的干细胞池。我们假设,受者体内移植供体细胞的复制压力增加,可能导致克隆造血(CH)变体不成比例地增殖。我们从 16 对相关的供体-受体(包括世界上存活时间最长的造血干细胞移植受体)中获取了血液样本,这些供体-受体在造血干细胞移植后的中位数为 33.8 年(范围:6.6 至 45.7)。在 16 对亲属中,有 11 对亲属在接受造血干细胞移植时的供体样本可供比较。我们对在髓系恶性肿瘤和CH中反复发生突变的基因进行了超灵敏双链测序,并对一组代表整个人类基因组内容的功能中性基因组区域进行了测序。在所有供体中都观察到了 CH 变异,甚至包括年仅 12 岁的供体。在有供体血液透析前样本的情况下,供体与接受者血液透析后的平均突变率相似(分别为每年2.0%和2.6%),都是在髓系恶性肿瘤中经常发生突变的基因中。HCT后配对供体和受体共有的393个变异中,有22个(5.6%)在受体中的变异等位基因频率(VAF)≥10倍。HCT 后的时间越长,受者的共享变异等位基因频率越高。总之,即使在造血干细胞移植后几十年,移植细胞中似乎也没有出现广泛的加速克隆扩增,这凸显了人类造血系统巨大的再生能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


