Oncometabolites at the crossroads of genetic, epigenetic and ecological alterations in cancer
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
By the time a tumor reaches clinical detectability, it contains around 108–109 cells. However, during tumor formation, significant cell loss occurs due to cell death. In some estimates, it could take up to a thousand cell generations, over a ~ 20-year life-span of a tumor, to reach clinical detectability, which would correspond to a “theoretical” generation of ~1030 cells. These rough calculations indicate that cancers are under negative selection. The fact that they thrive implies that they “evolve”, and that their evolutionary trajectories are shaped by the pressure of the environment. Evolvability of a cancer is a function of its heterogeneity, which could be at the genetic, epigenetic, and ecological/microenvironmental levels [1]. These principles were summarized in a proposed classification in which Evo (evolutionary) and Eco (ecological) indexes are used to label cancers [1]. The Evo index addresses cancer cell-autonomous heterogeneity (genetic/epigenetic). The Eco index describes the ecological landscape (non-cell-autonomous) in terms of hazards to cancer survival and resources available. The reciprocal influence of Evo and Eco components is critical, as it can trigger self-sustaining loops that shape cancer evolvability [2]. Among the various hallmarks of cancer [3], metabolic alterations appear unique in that they intersect with both Evo and Eco components. This is partly because altered metabolism leads to the accumulation of oncometabolites. These oncometabolites have traditionally been viewed as mediators of non-cell-autonomous alterations in the cancer microenvironment. However, they are now increasingly recognized as inducers of genetic and epigenetic modifications. Thus, oncometabolites are uniquely positioned at the crossroads of genetic, epigenetic and ecological alterations in cancer. In this review, the mechanisms of action of oncometabolites will be summarized, together with their roles in the Evo and Eco phenotypic components of cancer evolvability. An evolutionary perspective of the impact of oncometabolites on the natural history of cancer will be presented.
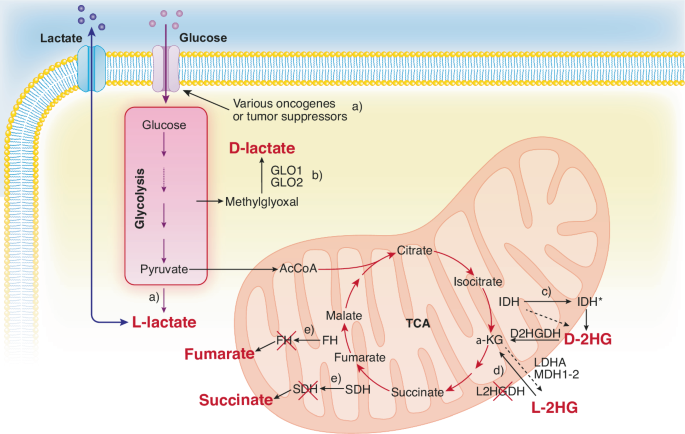
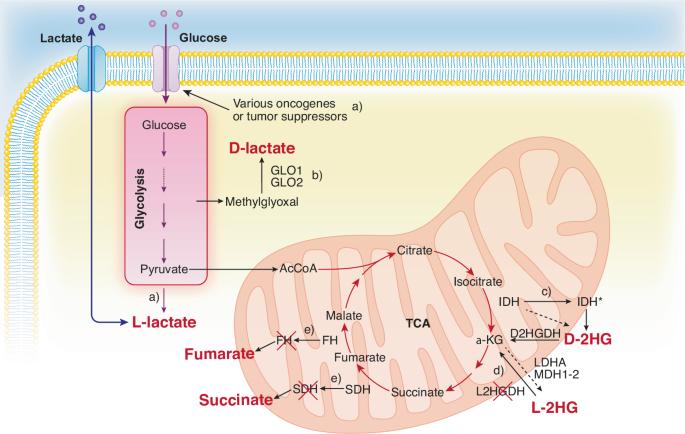
处于癌症遗传、表观遗传和生态改变交叉口的肿瘤代谢物
当肿瘤达到临床可检测的程度时,它大约包含 108-109 个细胞。然而,在肿瘤形成过程中,由于细胞死亡,细胞会大量丢失。据估计,在肿瘤约 20 年的生命周期中,可能需要经过多达一千代细胞才能达到临床可检测性,这相当于 "理论上 "产生了约 1030 个细胞。这些粗略的计算表明,癌症处于负选择状态。它们能茁壮成长这一事实意味着它们在 "进化",它们的进化轨迹是由环境压力决定的。癌症的可进化性是其异质性的函数,异质性可以是遗传、表观遗传和生态/微环境层面的[1]。这些原则被归纳为一种拟议的分类方法,其中 Evo(进化)和 Eco(生态)指数被用来标记癌症[1]。Evo 指数涉及癌细胞自主异质性(遗传/表观遗传)。生态指数从对癌症生存的危害和可用资源的角度描述生态景观(非细胞自主性)。Evo 和 Eco 成分的相互影响至关重要,因为它可以触发形成癌症可进化性的自我维持循环[2]。在癌症的各种特征中[3],代谢改变似乎是独一无二的,因为它们与 Evo 和 Eco 成分都有交集。这部分是因为代谢改变会导致副代谢物的积累。传统上,人们一直将这些癌上代谢物视为癌症微环境中非细胞自主改变的介质。然而,现在人们越来越认识到它们是遗传和表观遗传修饰的诱导物。因此,癌上代谢物处于癌症遗传、表观遗传和生态改变的交叉口,具有独特的地位。本综述将总结本体代谢物的作用机制,以及它们在癌症可进化性的 Evo 和 Eco 表型中的作用。此外,还将从进化的角度阐述肿瘤代谢物对癌症自然史的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


