In-Depth Thermodynamic and Kinetic Analysis of Ethane Diffusion in ZIF-8
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Flexible microporous ZIF-8 crystals show excellent separation behavior of small molecules such as ethaneand ethene. As such, hydrocarbon diffusion plays an essential role in the performance of these materials, yet determining accurate diffusion constants is nontrivial. Both ab initio and force-field based molecular dynamics simulations, coupled with umbrella sampling are applied in this work to characterize the diffusion of ethane in ZIF-8. Diffusion constants are extracted from the simulations by a combination of transition state theory and a random-walk hopping model, and are compared against experimentally measured values from literature. Ethane diffusion is a hindered process characterized by a transition state corresponding to an ethane molecule crossing the gate in between two neighboring cages formed by methylimidazole linkers. Free energy profiles of the diffusion process are derived and analyzed revealing the entropic nature of the barrier due to a counteracting of covalent host deformation energy and nonbonding host–guest interaction. A temperature analysis further confirms the entropic nature of the barrier and reveals an increased gate opening at increasing temperature. Finally, the loading dependency of diffusion is investigated revealing that increasing the ethane loading of the cages slightly slows down diffusion as a result of beneficial guest–guest interactions in the cages. Our findings yield essential elementary insight into how different molecular interactions influence the diffusion path of hydrocarbons throughout ZIF-8 crystals.
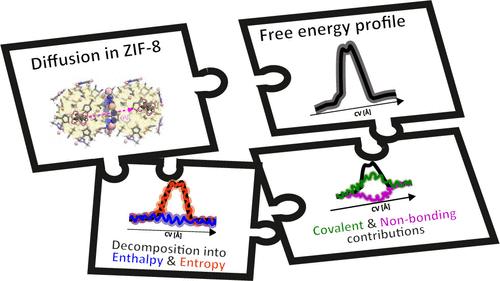
ZIF-8 中乙烷扩散的深度热力学和动力学分析
柔性微孔 ZIF-8 晶体对乙烷和乙烯等小分子具有出色的分离性能。因此,碳氢化合物的扩散对这些材料的性能起着至关重要的作用,然而确定准确的扩散常数并非易事。在这项工作中,我们应用了基于ab initio和力场的分子动力学模拟,并结合伞状采样来描述乙烷在ZIF-8中的扩散特性。通过结合过渡态理论和随机漫步跳跃模型,从模拟中提取了扩散常数,并与文献中的实验测量值进行了比较。乙烷扩散是一个受阻过程,其特征是乙烷分子穿过由甲基咪唑连接体形成的两个相邻笼子之间的栅极。对扩散过程的自由能曲线进行了推导和分析,揭示了由于共价宿主变形能和非键宿主-宿主相互作用的抵消作用而产生的障碍的熵性。温度分析进一步证实了势垒的熵性,并揭示了温度升高时栅极开度的增加。最后,对扩散的负载依赖性进行了研究,结果表明,由于笼子中有益的主宾相互作用,增加笼子中的乙烷负载会略微减缓扩散。我们的研究结果为了解不同的分子相互作用如何影响碳氢化合物在 ZIF-8 晶体中的扩散路径提供了重要的基本见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


