Potential effect of immediate postpartum use of injectable contraception on lactogenesis
IF 2.3
2区 医学
Q1 OBSTETRICS & GYNECOLOGY
引用次数: 0
Abstract
Objectives
We evaluated the effect of immediate postpartum use of depot medroxyprogesterone acetate (DMPA) on the timing of lactogenesis stage II (LS-II).
Study design
The initial design randomly assigned adults who delivered a full-term infant in 2019–2021 to receive within 48 hours of delivery: (1) DMPA, (2) placebo injection, or (3) no injection. Due to low enrollment, we changed in 2021–2023 to a nonrandomized design using matching at recruitment for obesity and delivery method and propensity score weighting for analysis. We combined data from both designs to compare immediate postpartum DMPA use (N = 55) vs control (placebo or no injection) group (N = 95). We defined noninferiority a priori as being met if the upper bound of a two-sided 95% CI for mean difference in time to LS-II between groups was <6 hours.
Results
The unweighted mean time to LS-II was 57.8 hours in the DMPA group (SD, 29.4) and 64.1 hours in the control group (SD, 36.1). Using propensity score weighting to make the groups comparable with respect to age, race, delivery method, and previous live births, the mean time to LS-II was 5.5 hours shorter (95% CI, −16.4, 5.5) for women in the DMPA relative to control group.
Conclusions
We found no evidence that DMPA use inhibits the onset of LS-II. Findings support immediate postpartum DMPA initiation among those intending to engage in human milk feeding.
Implications
A controlled trial (N = 150) did not detect any difference in time to lactogenesis stage II (“milk let-down”) between injectable contraception use within the first 48 hours postpartum and those without this exposure.
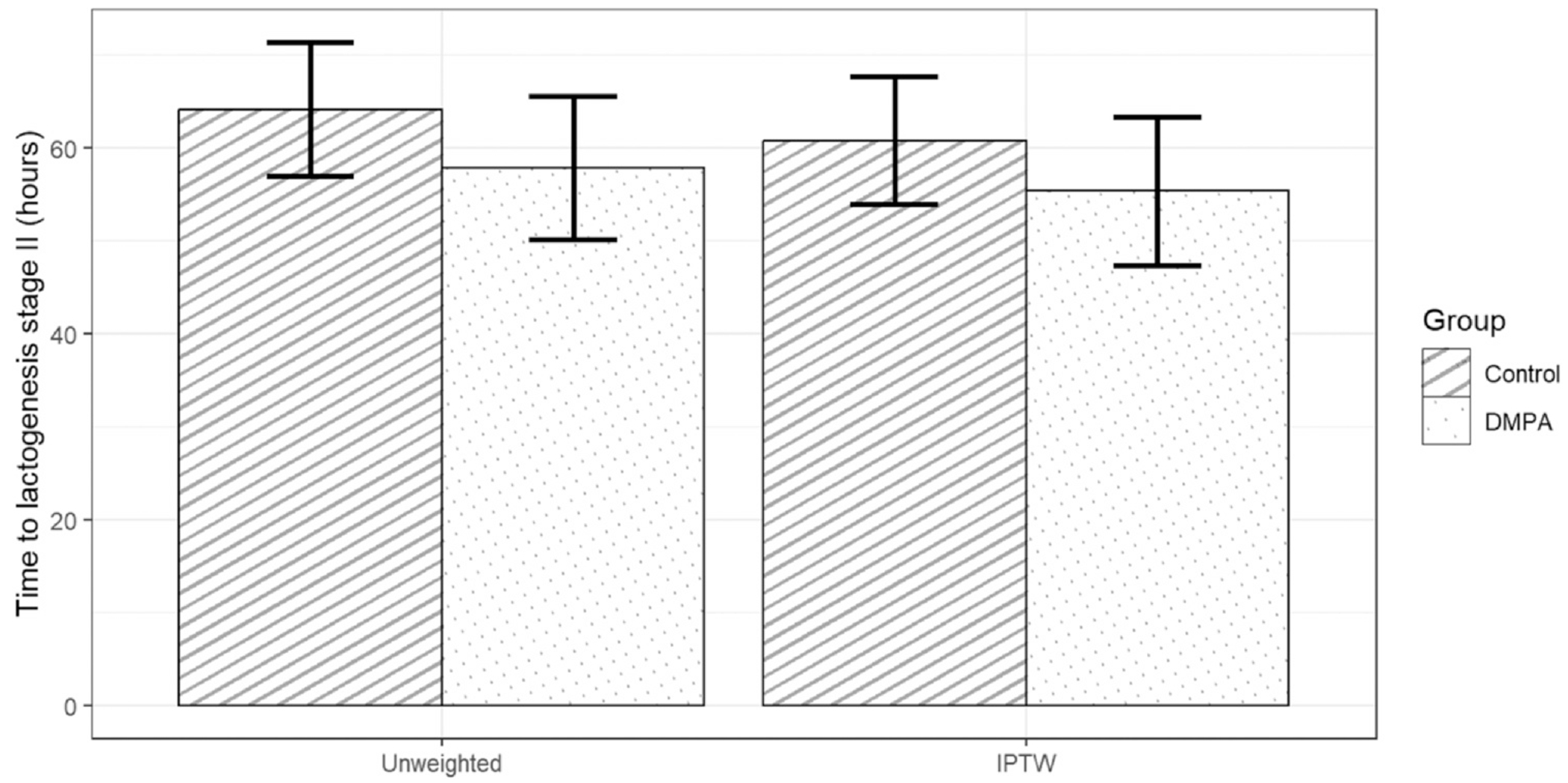
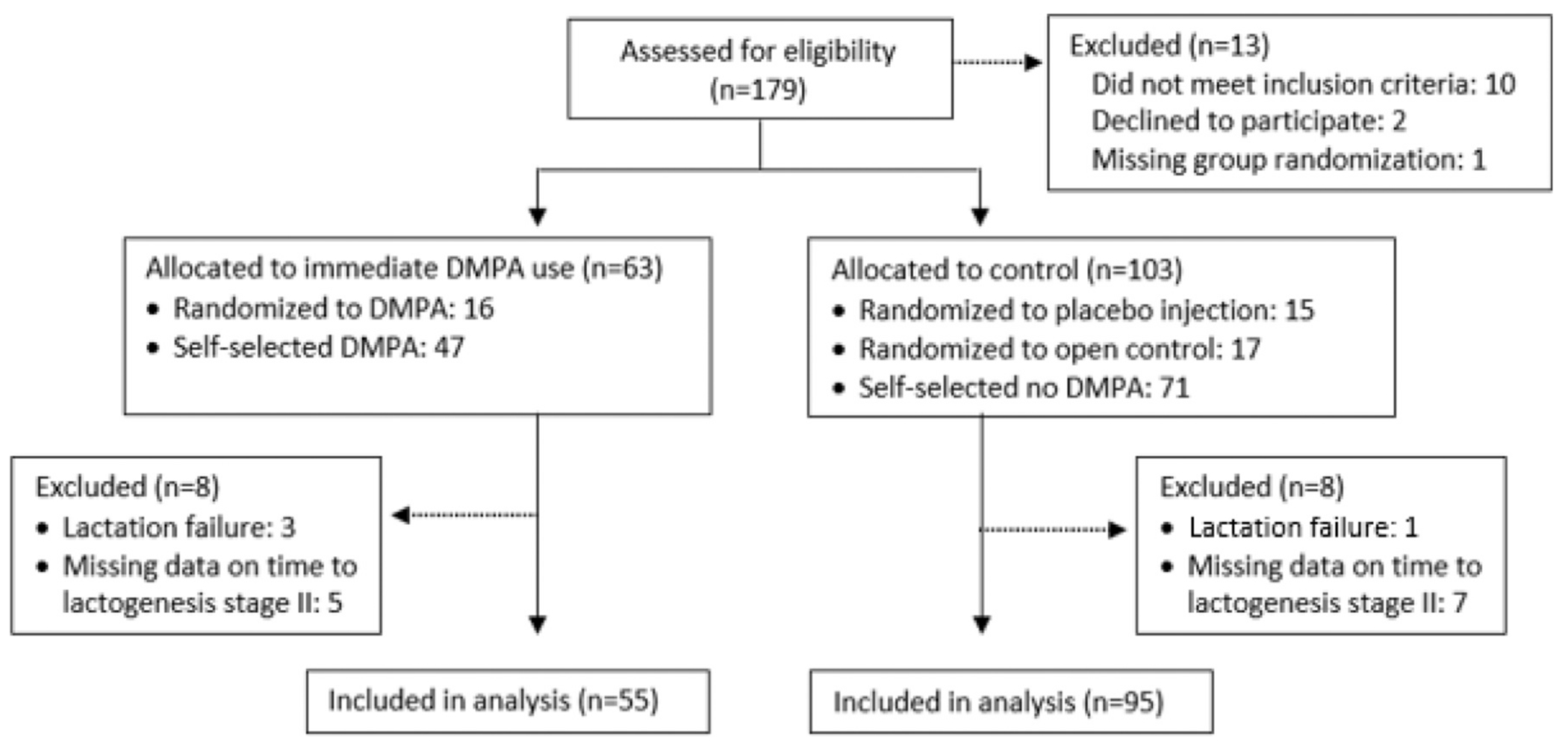
产后立即使用注射避孕药对泌乳的潜在影响。
研究目的我们评估了产后立即使用醋酸甲羟孕酮(DMPA)对泌乳期第二阶段(LS-II)时间的影响:最初的设计是随机分配在 2019-2021 年分娩足月婴儿的成人在分娩后 48 小时内接受:1)DMPA;2)安慰剂注射;或 3)不注射。由于注册人数较少,我们在 2021-2023 年改为非随机设计,在招募时对肥胖和分娩方式进行匹配,并采用倾向得分加权法进行分析。我们合并了两种设计的数据,对产后立即使用 DMPA 组(N=55)与对照组(安慰剂或不注射)(N=95)进行了比较。如果两组间 LS-II 平均差异时间的双侧 95% 置信区间 (CI) 上限为 "结果",我们就先验地将其定义为 "非劣效性":DMPA 组的 LS-II 非加权平均时间为 57.8 小时(标度为 29.4),对照组为 64.1 小时(标度为 36.1)。使用倾向得分加权法使各组在年龄、种族、分娩方式和既往活产情况方面具有可比性,DMPA 组妇女的 LS-II 平均时间比对照组缩短了 5.5 小时(95% CI,-16.4,5.5):结论:我们没有发现使用 DMPA 会抑制 LS-II 的发生。结论:我们没有发现使用 DMPA 会抑制 LS-II 的发生。研究结果支持那些打算母乳喂养的妇女在产后立即开始使用 DMPA:一项对照试验(N=150)未发现产后 48 小时内使用注射避孕药与未使用注射避孕药的产妇在泌乳期第二阶段("下奶")的时间上有任何差异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Contraception
医学-妇产科学
CiteScore
4.70
自引率
17.20%
发文量
211
审稿时长
69 days
期刊介绍:
Contraception has an open access mirror journal Contraception: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
The journal Contraception wishes to advance reproductive health through the rapid publication of the best and most interesting new scholarship regarding contraception and related fields such as abortion. The journal welcomes manuscripts from investigators working in the laboratory, clinical and social sciences, as well as public health and health professions education.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


