Mechanotransduction pathways regulating YAP nuclear translocation under Yoda1 and vibration in osteocytes
IF 3.5
2区 医学
Q2 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Yes-associated protein (YAP) is a mechanosensitive protein crucial for bone remodeling. Although research has identified pathways and components involved in YAP regulation, the precise mechanisms of its localization during Piezo1 activation or vibration remain unclear. Piezo1, a mechanosensitive ion channel, allows calcium ions to flow into cells upon activation. Recent studies suggest that combining Yoda1, a Piezo1 activator, with low-magnitude high-frequency (LMHF) vibration (>30 Hz, <1 g acceleration) enhances YAP nuclear translocation. This combination potentially improves the mechanoresponse and therapeutic efficacy of LMHF vibration in bone cells. This study aims to elucidate how Yoda1 and LMHF vibration regulate mechanosensitive structures and pathways, leading to YAP nuclear translocation in MLO-Y4 osteocyte like cells. We investigated the roles of the cytoskeleton and nuclear envelope (NE) in YAP activation under combined LMHF vibration and Yoda1 treatments. Additionally, we analyzed differentially expressed genes (DEGs) in MLO-Y4 cells subjected to these treatments and in Piezo1 knockdown MLO-Y4 cells exposed to vibration. Our findings indicated that increased YAP nuclear translocation with combined treatment may result from the distinct effects of Yoda1 and vibration. Specifically, Yoda1 influenced YAP through mechanisms involving actin and NE dynamics, while LMHF vibration may modulate YAP via the interleukin 6 (IL6)/signal transducer and activator of transcription 3 (STAT3) axis. This study provides new insights and potential therapeutic targets for osteocyte-related pathologies.
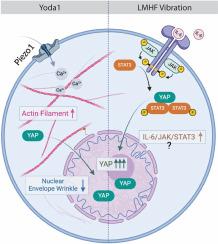
调节 Yoda1 和振动作用下骨细胞中 YAP 核转位的机制传导途径
是相关蛋白(YAP)是一种机械敏感蛋白,对骨重塑至关重要。尽管研究发现了参与 YAP 调节的途径和成分,但其在 Piezo1 激活或振动过程中定位的确切机制仍不清楚。Piezo1 是一种机械敏感性离子通道,可使钙离子在激活时流入细胞。最近的研究表明,将 Piezo1 激活剂 Yoda1 与低幅高频(LMHF)振动(>30 Hz、
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bone
医学-内分泌学与代谢
CiteScore
8.90
自引率
4.90%
发文量
264
审稿时长
30 days
期刊介绍:
BONE is an interdisciplinary forum for the rapid publication of original articles and reviews on basic, translational, and clinical aspects of bone and mineral metabolism. The Journal also encourages submissions related to interactions of bone with other organ systems, including cartilage, endocrine, muscle, fat, neural, vascular, gastrointestinal, hematopoietic, and immune systems. Particular attention is placed on the application of experimental studies to clinical practice.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


