Obesity-induced extracellular vesicles proteins drive the endometrial cancer pathogenesis: therapeutic potential of HO-3867 and Metformin
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
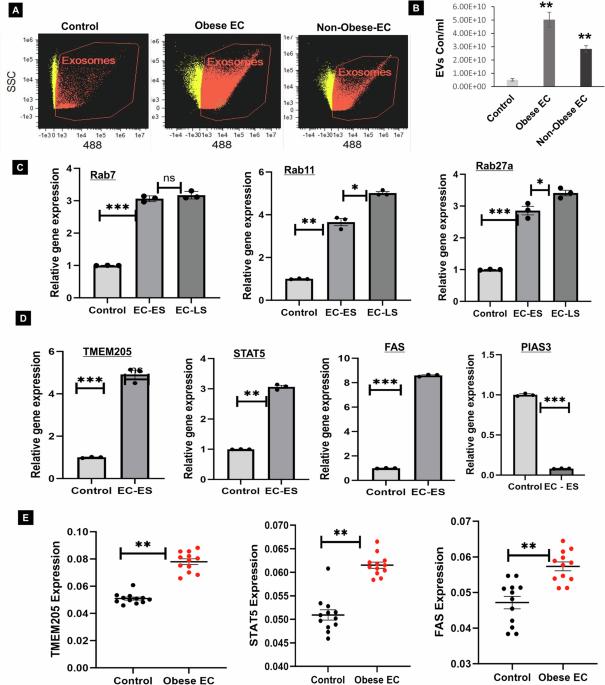
Endometrial cancer (EC) is the leading gynecologic malignancy in the United States with obesity implicated in 57% of cases. This research investigates the molecular complexities of extracellular vesicles (EV) secretion as carriers of oncogenic protein and their involvement in obesity-mediated EC. An understanding of these mechanisms is pivotal for unraveling pathways relevant to obesity-associated EC, thereby guiding the development of innovative prevention and treatment strategies. Our exploration revealed a significant increase in EV secretion carrying oncogenic proteins (TMEM205, STAT5, and FAS) in adipose and uterine tissues/serum samples from obese EC patients compared to control (without cancer). We identified alterations in EV-regulating proteins (Rab7, Rab11, and Rab27a) in obesity-mediated EC patients, adipose/uterine tissues, and serum samples. Through a 24-week analysis of the effects of a 45% kcal high-fat diet (HFD) on mice, we observed increased body weight, increased adipose tissue, enlarged uterine horns, and increased inflammation in the HFD group. This correlated with elevated levels of EV secretion and increased expression of oncogenic proteins TMEM205, FAS, and STAT5 and downregulation of the tumor suppressor gene PIAS3 in adipose and uterine tissues. Furthermore, our study confirmed that adipocyte derived EV increased EC cell proliferation, migration and xenograft tumor growth. Additionally, we identified that the small molecule inhibitors (HO-3867) or Metformin inhibited EV secretion in vitro and in vivo, demonstrating significant inhibition of high glucose or adipocyte-mediated EC cell proliferation and a reduction in body weight and adipose tissue accumulation when administered to HFD mice. Moreover, HO-3867 or Metformin treatment inhibited HFD induced hyperplasia (precursor of EC) by altering the expression of EV-regulated proteins and decreasing oncogenic protein expression levels. This study provides critical insights into the mechanisms underpinning obesity-mediated EV secretion with oncogenic protein expression, shedding light on their role in EC pathogenesis. Additionally, it offers pre-clinical evidence supporting the initiation of novel studies for EV-targeted therapies aimed at preventing obesity-mediated EC.
肥胖诱导的细胞外囊泡蛋白驱动子宫内膜癌发病机制:HO-3867 和二甲双胍的治疗潜力
子宫内膜癌(EC)是美国最主要的妇科恶性肿瘤,其中 57% 的病例与肥胖有关。这项研究调查了细胞外囊泡(EV)分泌作为致癌蛋白载体的分子复杂性及其在肥胖介导的子宫内膜癌中的参与。了解这些机制对于揭示肥胖相关EC的相关途径至关重要,从而指导创新性预防和治疗策略的开发。我们的研究发现,与对照组(未患癌症)相比,肥胖EC患者的脂肪和子宫组织/血清样本中携带致癌蛋白(TMEM205、STAT5和FAS)的EV分泌明显增加。我们在肥胖引发的EC患者、脂肪/子宫组织和血清样本中发现了EV调节蛋白(Rab7、Rab11和Rab27a)的变化。通过对 45% 千卡高脂饮食(HFD)对小鼠影响的 24 周分析,我们观察到 HFD 组小鼠体重增加、脂肪组织增加、子宫角增大、炎症加重。这与脂肪组织和子宫组织中 EV 分泌水平升高、致癌蛋白 TMEM205、FAS 和 STAT5 表达增加以及抑癌基因 PIAS3 下调有关。此外,我们的研究证实,脂肪细胞衍生的 EV 增加了 EC 细胞的增殖、迁移和异种移植肿瘤的生长。此外,我们发现小分子抑制剂(HO-3867)或二甲双胍可抑制体外和体内的EV分泌,对高血糖或脂肪细胞介导的EC细胞增殖有显著抑制作用,并可减少高血糖小鼠的体重和脂肪组织堆积。此外,HO-3867 或二甲双胍通过改变 EV 调控蛋白的表达和降低致癌蛋白的表达水平,抑制了 HFD 诱导的增生(EC 的前体)。这项研究深入揭示了肥胖介导的EV分泌和致癌蛋白表达的机制,阐明了它们在EC发病机制中的作用。此外,它还提供了临床前证据,支持启动旨在预防肥胖介导的心肌梗死的 EV 靶向疗法的新型研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


