Protein kinase D2 modulates hepatic insulin sensitivity in male mice
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objectives
Protein kinase D (PKD) family is emerging as relevant regulator of metabolic homeostasis. However, the precise role of PKD2 in modulating hepatic insulin signaling has not been fully elucidated and it is the aim of this study.
Methods
PKD inhibition was analyzed for insulin signaling in mouse and human hepatocytes. PKD2 was overexpressed in Huh7 hepatocytes and mouse liver, and insulin responses were evaluated. Mice with hepatocyte-specific PKD2 depletion (PKD2ΔHep) and PKD2fl/fl mice were fed a chow (CHD) or high fat diet (HFD) and glucose homeostasis and lipid metabolism were investigated.
Results
PKD2 silencing enhanced insulin signaling in hepatocytes, an effect also found in primary hepatocytes from PKD2ΔHep mice. Conversely, a constitutively active PKD2 mutant reduced insulin-stimulated AKT phosphorylation. A more in-depth analysis revealed reduced IRS1 serine phosphorylation under basal conditions and increased IRS1 tyrosine phosphorylation in PKD2ΔHep primary hepatocytes upon insulin stimulation and, importantly PKD co-immunoprecipitates with IRS1. In vivo constitutively active PKD2 overexpression resulted in a moderate impairment of glucose homeostasis and reduced insulin signaling in the liver. On the contrary, HFD-fed PKD2ΔHep male mice displayed improved glucose and pyruvate tolerance, as well as higher peripheral insulin tolerance and enhanced hepatic insulin signaling compared to control PKD2fl/fl mice. Despite of a remodeling of hepatic lipid metabolism in HFD-fed PKD2ΔHep mice, similar steatosis grade was found in both genotypes.
Conclusions
Results herein have unveiled an unknown role of PKD2 in the control of insulin signaling in the liver at the level of IRS1 and point PKD2 as a therapeutic target for hepatic insulin resistance.
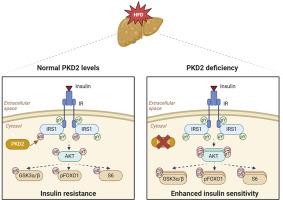
蛋白激酶 D2 可调节雄性小鼠肝脏对胰岛素的敏感性。
目的:蛋白激酶D(PKD)家族正在成为代谢平衡的相关调节因子。然而,PKD2 在调节肝脏胰岛素信号传导中的确切作用尚未完全阐明,这正是本研究的目的所在:方法:在小鼠和人类肝细胞中分析了 PKD 抑制胰岛素信号转导的情况。在 Huh7 肝细胞和小鼠肝脏中过表达 PKD2,并评估胰岛素反应。给肝细胞特异性PKD2缺失的小鼠(PKD2ΔHep)和PKD2fl/fl小鼠喂食饲料(CHD)或高脂饮食(HFD),并研究葡萄糖稳态和脂质代谢:结果:PKD2沉默增强了肝细胞中的胰岛素信号转导,在PKD2ΔHep小鼠的原代肝细胞中也发现了这种效应。相反,组成型活性 PKD2 突变体降低了胰岛素刺激的 AKT 磷酸化。更深入的分析显示,PKD2ΔHep原代肝细胞在基础条件下IRS1丝氨酸磷酸化减少,而在胰岛素刺激下IRS1酪氨酸磷酸化增加,重要的是PKD与IRS1共沉淀。体内组成型活性 PKD2 过表达会导致肝脏中葡萄糖稳态中度受损和胰岛素信号转导减少。相反,与对照组PKD2fl/fl小鼠相比,喂食高氟日粮的PKD2ΔHep雄性小鼠显示出更好的葡萄糖和丙酮酸耐受性,以及更高的外周胰岛素耐受性和更强的肝脏胰岛素信号传导。尽管高密度脂蛋白饲料喂养的PKD2ΔHep小鼠的肝脏脂质代谢发生了重塑,但两种基因型小鼠的脂肪变性程度相似:本文的研究结果揭示了PKD2在控制肝脏IRS1水平的胰岛素信号传导中的未知作用,并指出PKD2是肝脏胰岛素抵抗的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


