FITM2 deficiency results in ER lipid accumulation, ER stress, and reduced apolipoprotein B lipidation and VLDL triglyceride secretion in vitro and in mouse liver
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
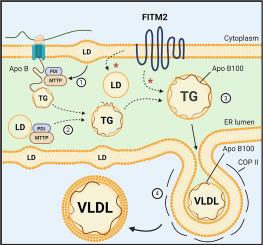
Triglycerides (TGs) associate with apolipoprotein B100 (apoB100) to form very low density lipoproteins (VLDLs) in the liver. The repertoire of factors that facilitate this association is incompletely understood. FITM2, an integral endoplasmic reticulum (ER) protein, was originally discovered as a factor participating in cytosolic lipid droplet (LD) biogenesis in tissues that do not form VLDL. We hypothesized that in the liver, in addition to promoting cytosolic LD formation, FITM2 would also transfer TG from its site of synthesis in the ER membrane to nascent VLDL particles within the ER lumen.
Methods
Experiments were conducted using a rat hepatic cell line (McArdle-RH7777, or McA cells), an established model of mammalian lipoprotein metabolism, and mice. FITM2 expression was reduced using siRNA in cells and by liver specific cre-recombinase mediated deletion of the Fitm2 gene in mice. Effects of FITM2 deficiency on VLDL assembly and secretion in vitro and in vivo were measured by multiple methods, including density gradient ultracentrifugation, chromatography, mass spectrometry, stimulated Raman scattering (SRS) microscopy, sub-cellular fractionation, immunoprecipitation, immunofluorescence, and electron microscopy.
Main findings
1) FITM2-deficient hepatic cells in vitro and in vivo secrete TG-depleted VLDL particles, but the number of particles is unchanged compared to controls; 2) FITM2 deficiency in mice on a high fat diet (HFD) results in decreased plasma TG levels. The number of apoB100-containing lipoproteins remains similar, but shift from VLDL to low density lipoprotein (LDL) density; 3) Both in vitro and in vivo, when TG synthesis is stimulated and FITM2 is deficient, TG accumulates in the ER, and despite its availability this pool is unable to fully lipidate apoB100 particles; 4) FITM2 deficiency disrupts ER morphology and results in ER stress.
Conclusion
The results suggest that FITM2 contributes to VLDL lipidation, especially when newly synthesized hepatic TG is in abundance. In addition to its fundamental importance in VLDL assembly, the results also suggest that under dysmetabolic conditions, FITM2 may be an important factor in the partitioning of TG between cytosolic LDs and VLDL particles.
FITM2 缺乏会导致体外和小鼠肝脏中的ER脂质积累、ER应激、载脂蛋白B脂化和VLDL甘油三酯分泌减少。
目的:甘油三酯(TGs)与载脂蛋白 B100(apoB100)结合,在肝脏中形成极低密度脂蛋白(VLDLs)。目前还不完全清楚促进这种结合的一系列因素。FITM2是一种完整的内质网(ER)蛋白,最初是作为一种参与细胞膜脂滴(LD)生物生成的因子在不形成VLDL的组织中被发现的。我们假设,在肝脏中,FITM2 除了促进细胞膜脂滴的形成外,还能将 TG 从其在 ER 膜上的合成部位转移到 ER 腔内的新生 VLDL 颗粒中:实验使用大鼠肝细胞系(McArdle-RH7777,或 McA 细胞)(哺乳动物脂蛋白代谢的成熟模型)和小鼠进行。在细胞中使用 siRNA 减少 FITM2 的表达,在小鼠中使用肝脏特异性 cre-recombinase 介导的 Fitm2 基因缺失。通过多种方法,包括密度梯度超速离心、色谱法、质谱法、刺激拉曼散射(SRS)显微镜、亚细胞分馏、免疫沉淀、免疫荧光和电子显微镜,测定了 FITM2 缺乏对体外和体内 VLDL 组装和分泌的影响:主要发现:1)体外和体内 FITM2 缺乏的肝细胞分泌去TG的 VLDL 颗粒,但颗粒数量与对照组相比没有变化;2)高脂饮食(HFD)小鼠 FITM2 缺乏导致血浆 TG 水平下降。含载脂蛋白 B100 的脂蛋白数量保持相似,但密度从 VLDL 转向低密度脂蛋白(LDL);3)在体外和体内,当 TG 合成受到刺激而 FITM2 缺乏时,TG 在 ER 中积累,尽管可以获得 TG,但该库无法完全脂化载脂蛋白 B100 颗粒;4)FITM2 缺乏会破坏 ER 形态并导致 ER 应激:研究结果表明,FITM2 有助于 VLDL 脂化,尤其是在新合成的肝脏 TG 大量存在时。除了在 VLDL 组装过程中发挥重要作用外,研究结果还表明,在代谢紊乱条件下,FITM2 可能是在细胞膜低密度脂蛋白和 VLDL 颗粒之间分配 TG 的一个重要因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


