Inhibition of proteasome activity facilitates definitive endodermal specification of pluripotent stem cells by influencing YAP signalling
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
The knowledge of the molecular players that regulate the generation of endoderm cells is imperative to obtain homogenous population of pancreatic β-cells from stem cells. The Ubiquitin proteasome system (UPS) has been envisaged as a crucial intracellular protein degradation system, but its role in the generation of β-cells remains elusive. Hence, it would be appropriate to unravel the potential role of UPS in endoderm specification and utilize the understanding to generate β-cells from pluripotent stem cells.
Materials and methods
The pluripotent stem cells (mESCs, miPSCs and hIPSCs) were subjected to differentiation towards pancreatic β-cells and assessed the proteasomal activity during endodermal differentiation. Pharmacologic agents MG132 and IU-1 were employed to inhibit and activate proteasomal activity respectively at the definitive endoderm stage to investigate its impact on the generation of β-cells. The expression of stage-specific genes were analyzed at transcript and protein levels. We also explored the role of unfolded protein response and UPS-regulated signalling pathways in endodermal differentiation.
Key findings
We observed decreased proteasomal activity specifically during endoderm, but not during the generation of other lineages. Extraneous proteasomal inhibition enhanced the expression of endodermal genes while increasing the proteasomal activity hindered definitive endodermal differentiation. Proteasomal inhibition at the definitive endodermal stage culminated in an enriched generation of insulin-positive cells. Elevated endodermal gene expression was consistent in mESCs and hIPSCs upon proteasomal inhibition. Mechanistic insight revealed the proteasome-inhibited enhanced endodermal differentiation to be via modulating the YAP pathway.
Significance
Our study unravels the specific involvement of UPS in endoderm cell generation from pluripotent stem cells and paves the way for obtaining potential definitive endodermal cells for plausible cellular therapy in the future.
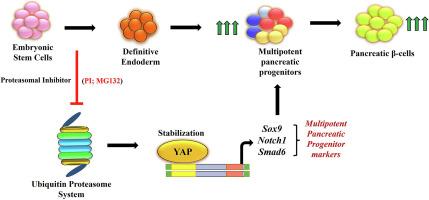
抑制蛋白酶体活性可通过影响 YAP 信号促进多能干细胞的最终内胚层分化。
目的:要从干细胞中获得同质的胰腺β细胞,就必须了解调控内胚层细胞生成的分子角色。泛素蛋白酶体系统(UPS)一直被认为是细胞内蛋白质降解的关键系统,但它在β细胞生成过程中的作用仍然难以捉摸。因此,揭示UPS在内胚层规格化中的潜在作用,并利用这一认识从多能干细胞中生成β细胞是合适的:多能干细胞(mESCs、miPSCs和hIPSCs)向胰腺β细胞分化,并评估内胚层分化过程中蛋白酶体的活性。在终末内胚层阶段,采用药剂 MG132 和 IU-1 分别抑制和激活蛋白酶体活性,以研究其对β细胞生成的影响。我们在转录本和蛋白质水平上分析了阶段特异性基因的表达。我们还探讨了未折叠蛋白反应和 UPS 调节的信号通路在内皮分化中的作用:主要发现:我们观察到蛋白酶体活性在内耳分化过程中降低,而在其他细胞系分化过程中没有降低。外来的蛋白酶体抑制增强了内胚层基因的表达,而蛋白酶体活性的增强则阻碍了最终内胚层的分化。在最终内胚层阶段抑制蛋白酶体,最终会产生大量胰岛素阳性细胞。蛋白酶体抑制后,mESCs 和 hIPSCs 的内胚层基因表达一致升高。从机制上看,蛋白酶体抑制的内胚层分化增强是通过调节YAP通路实现的:我们的研究揭示了UPS在多能干细胞生成内胚层细胞过程中的特殊参与,为将来获得潜在的确定性内胚层细胞用于可行的细胞疗法铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


