Phosphoproteomic analysis reveals the mechanisms of human umbilical cord mesenchymal stem cell-derived exosomes attenuate renal aging
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
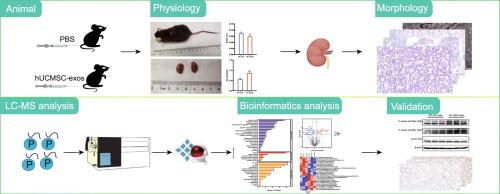
Aging is a critical biological process, with particularly notable impacts on the kidneys. Exosomes derived from human umbilical cord mesenchymal stem cells (hUC-MSCs) are capable of transferring various bioactive molecules, which exhibit beneficial therapeutic effects on kidney diseases. This study demonstrates that exosomes derived from hUC-MSCs ameliorate cellular senescence in the kidneys of naturally aging mice. These exosomes reduce the protein expression of senescence markers and senescence-associated secretory phenotypes (SASP) leading to fewer DNA damage foci and increased expression of the proliferation indicator Ki67. During the aging process, many proteins undergo phosphorylation modifications. We utilized data-independent acquisition (DIA) phosphoproteomics to study kidneys of naturally aging mice and those treated with hUC-MSC-derived exosomes. We observed elevated phosphorylation levels of the differentially phosphorylated proteins, Lamin A/C, at Ser390 and Ser392 sites, which were subsequently verified by western blotting. Overall, this study provides a new molecular characterization of hUC-MSC-derived exosomes in mitigating cellular senescence in the kidneys.
Significance
DIA phosphoproteomics was employed to investigate phosphorylated proteins in the kidney tissues of naturally aging mice with hUCMSC-exos treated. The results demonstrated that the DIA technique detected a higher abundance of phosphorylated proteins. We identified 24 significantly differentially phosphorylated proteins, and found that the phosphorylation of specific Lamin A/C sites is crucial for preventing cellular senescence. This study will help to better reveal the related phosphorylated proteins involved in hUCMSC-exos intervention in the kidneys of naturally aging mice, providing a foundation for future research on specific phosphorylation sites of proteins as potential therapeutic targets for renal aging-related diseases.
磷蛋白组分析揭示了人脐带间充质干细胞外泌体减轻肾脏衰老的机制
衰老是一个关键的生物过程,对肾脏的影响尤为显著。从人脐带间充质干细胞(hUC-MSCs)中提取的外泌体能够转移各种生物活性分子,对肾脏疾病具有有益的治疗作用。本研究证明,从人脐带间充质干细胞中提取的外泌体可改善自然衰老小鼠肾脏的细胞衰老。这些外泌体减少了衰老标志物和衰老相关分泌表型(SASP)的蛋白表达,导致DNA损伤灶减少,增殖指标Ki67的表达增加。在衰老过程中,许多蛋白质会发生磷酸化修饰。我们利用数据独立获取(DIA)磷酸化蛋白质组学研究了自然衰老小鼠的肾脏和用源自hUC-间充质干细胞的外泌体治疗的小鼠的肾脏。我们观察到不同磷酸化蛋白 Lamin A/C 在 Ser390 和 Ser392 位点的磷酸化水平升高,随后通过 Western 印迹进行了验证。总之,这项研究为 hUC 间充质干细胞衍生的外泌体在减轻肾脏细胞衰老方面提供了新的分子特征。意义:研究人员采用 DIA 磷酸化蛋白质组学研究了经 hUCMSC 外泌体处理的自然衰老小鼠肾脏组织中的磷酸化蛋白质。结果表明,DIA 技术能检测到更高丰度的磷酸化蛋白。我们发现了 24 种明显不同的磷酸化蛋白,并发现特定 Lamin A/C 位点的磷酸化对防止细胞衰老至关重要。这项研究将有助于更好地揭示参与 hUCMSC-exos 对自然衰老小鼠肾脏干预的相关磷酸化蛋白,为今后研究特定磷酸化位点蛋白作为肾脏衰老相关疾病的潜在治疗靶点奠定基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


