DSSBU: A novel mass spectrometry-cleavable analogue of the BS3 cross-linker
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
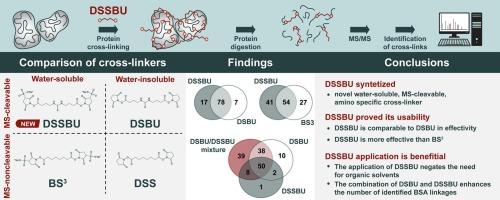
Protein cross-linking has assumed an irreplaceable role in structural proteomics. Recently, significant efforts have been made to develop novel mass spectrometry (MS)-cleavable reagents. At present, only water-insoluble MS-cleavable cross-linkers are commercially available. However, to comprehensively analyse the various chemical and structural motifs making up proteins, it is necessary to target different protein sites with varying degrees of hydrophilicity. Here we introduce the new MS-cleavable cross-linker disulfodisuccinimidyl dibutyric urea (DSSBU), which we have developed in-house for this purpose. DSSBU contains an N-hydroxysulfosuccinimide (sulfo-NHS) reactive group, so it can serve as a water-soluble counterpart to the widely used cross-linker disuccinimidyl dibutyric urea (DSBU). To investigate the applicability of DSSBU, we compared the efficacy of four similar cross-linkers: bis[sulfosuccinimidyl] suberate (BS3), disuccinimidyl suberate (DSS), DSBU and DSSBU with bovine serum albumin. In addition, we compared the efficacy of DSBU and DSSBU with human haemoglobin. Our results demonstrate that the sulfo-NHS group ensures the superior water solubility of DSSBU and thus negates the need for organic solvents such as dimethyl sulfoxide while preserving the effectivity of urea-based MS-cleavable crosslinkers such as DSBU. Additionally, it makes it possible to target polar regions in proteins. The data gathered are available via ProteomeXchange under identifier PXD055284.
Significance
We have synthesized the novel protein cross-linker DSSBU, which combines sulfo-NHS ester chemistry with a mass spectrometry-cleavable urea group. This makes DSSBU a water-soluble, MS-cleavable cross-linker that reacts with amino groups. To our knowledge, it is the first cross-linker which combines all three of these characteristics. We have tested the performance of our novel cross-linker on bovine serum albumin, a model widely used by the cross-linking mass spectrometry community, and on human haemoglobin. We have comprehensively assessed the performance of DSSBU and compared its efficacy with that of three other cross-linkers in current use (BS3, DSS and DSBU). We conclude that our novel cross-linker surpasses its MS-non-cleavable analogue BS3 in performance and that its water solubility eliminates the need for organic solvents while its hydrophilicity allows for the targetting of polar regions in proteins. Therefore, it will likely become a significant addition to the portfolio of N-hydroxysuccinimide ester cross-linkers.
DSSBU:BS3 交联剂的新型质谱可裂解类似物。
蛋白质交联在结构蛋白质组学中发挥着不可替代的作用。最近,人们大力开发新型质谱(MS)可溶解试剂。目前,市场上只有不溶于水的 MS 可溶解交联剂。然而,要全面分析构成蛋白质的各种化学和结构基团,就必须针对亲水性程度不同的蛋白质位点。在此,我们介绍了内部为此开发的新型 MS 可裂解交联剂二磺酸二琥珀酰亚胺基二丁基脲(DSSBU)。DSSBU 含有一个 N-羟基磺基琥珀酰亚胺(sulfo-NHS)反应基团,因此可以作为广泛使用的交联剂二琥珀酰亚胺基二丁基脲(DSBU)的水溶性对应物。为了研究 DSSBU 的适用性,我们比较了四种类似交联剂:双[磺基琥珀酰亚胺基]辛二酸酯(BS3)、双琥珀酰亚胺基辛二酸酯(DSS)、DSBU 和 DSSBU 与牛血清白蛋白的功效。此外,我们还比较了 DSBU 和 DSSBU 与人类血红蛋白的功效。我们的研究结果表明,磺基-NHS 基团可确保 DSSBU 具有优异的水溶性,因此无需使用二甲亚砜等有机溶剂,同时还能保持 DSBU 等脲基 MS 可裂解交联剂的功效。此外,这种方法还能瞄准蛋白质的极性区域。收集到的数据可通过蛋白质组交换(ProteomeXchange)获取,标识符为 PXD055284。意义:我们合成了新型蛋白质交联剂 DSSBU,它结合了磺基-NHS 酯化学和质谱可裂解脲基。这使得 DSSBU 成为一种可与氨基反应的水溶性、质谱可裂解交联剂。据我们所知,这是第一种兼具上述三种特性的交联剂。我们在牛血清白蛋白(交联质谱界广泛使用的模型)和人类血红蛋白上测试了新型交联剂的性能。我们全面评估了 DSSBU 的性能,并将其与目前使用的其他三种交联剂(BS3、DSS 和 DSBU)的功效进行了比较。我们的结论是,我们的新型交联剂在性能上超过了其 MS-非可溶解类似物 BS3,其水溶性使其无需使用有机溶剂,而其亲水性则使其可以靶向蛋白质中的极性区域。因此,它很可能成为 N-羟基琥珀酰亚胺酯交联剂产品组合的重要补充。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


