Insufficient S-sulfhydration of serum and glucocorticoid-regulated kinase 1 participates in hyperhomocysteinemia-induced liver injury
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background & aims
Previous studies have established that hyperhomocysteinemia (HHcy) significantly contributes to the development of non-alcoholic steatohepatitis (NASH). Conversely, hydrogen sulfide (H2S) has shown potential in mitigating NASH. Despite these findings, it remains uncertain whether H2S can serve as a therapeutic agent against HHcy-induced liver damage.
Methods
Mice were fed a high-methionine diet to induce HHcy and HepG2 cells were exposed to homocysteine (Hcy). In both models, we assessed liver injury, H2S concentration, and autophagy levels. For rescue, sodium hydrosulfide (NaHS), an H2S donor, was used to test its potential in reversing hepatic pathological features induced by HHcy.
Results
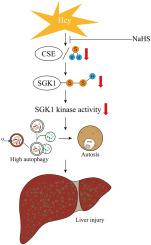
1) Hcy accumulation led to liver damage and increased autophagy. This was linked to insufficient S-sulfhydration of serum and glucocorticoid-regulated kinase 1 (SGK1) at Cys244 and Cys282, a crucial autophagy regulator. The deficiency in S-sulfhydration was resulted from downregulation of cystathionine-γ-lyase (CSE) and subsequent H2S decrease, leading to SGK1 inactivation. 2) Administration of NaHS reduced the liver damage caused by high Hcy levels and restored H2S levels, promoting the S-sulfhydration and activation of SGK1. 3) Pharmacological inhibition of SGK1 induced autosis, a specific type of cell death caused by overactivation of autophagy. Conversely, a constitutively active mutant of SGK1 (SGK1S422D) significantly decreased autophagy and improved cell viability.
Conclusions
NaHS supplementation mitigates HHcy-induced liver injury by downregulating hepatic autophagy through the S-sulfhydration and activation of SGK1. This post-translational modification by H2S holds promise as a therapeutic approach for HHcy-induced liver injury.
血清和糖皮质激素调控激酶 1 的 S-脱水不足参与高同型半胱氨酸血症诱发的肝损伤
背景和目的:先前的研究已经证实,高同型半胱氨酸血症(HHcy)是导致非酒精性脂肪性肝炎(NASH)的重要原因。相反,硫化氢(H2S)却显示出缓解 NASH 的潜力。尽管有这些发现,但硫化氢是否能作为一种治疗剂来对抗 HHcy 引起的肝损伤仍不确定:方法:给小鼠喂食高蛋氨酸饮食以诱导 HHcy,并将 HepG2 细胞暴露于同型半胱氨酸(Hcy)中。在这两种模型中,我们评估了肝损伤、H2S 浓度和自噬水平。为了进行挽救,我们使用了硫氢化钠(一种 H2S 供体)来测试其逆转 HHcy 诱导的肝脏病理特征的潜力:1)Hcy 的积累导致肝损伤和自噬增加。这与血清和糖皮质激素调节激酶 1(SGK1)在 Cys244 和 Cys282 上的 S-硫酸化不足有关,S-硫酸化是自噬的关键调节因子。胱硫醚-γ-赖氨酸酶(CSE)的下调和随后的 H2S 减少导致 SGK1 失活,从而导致 S-硫酸化不足。2)服用 NaHS 可减轻高 Hcy 对肝脏的损伤,恢复 H2S 水平,促进 S-硫酸化和 SGK1 的活化。3) 药物抑制 SGK1 可诱导自噬,这是一种因自噬过度激活而导致的特殊类型的细胞死亡。相反,SGK1的组成活性突变体(SGK1S422D)能显著降低自噬,提高细胞活力:结论:补充 NaHS 可通过 S-硫酸化和活化 SGK1 来下调肝脏自噬,从而减轻 HHcy 诱导的肝损伤。H2S的这种翻译后修饰有望成为HHcy诱导的肝损伤的一种治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


