Optineurin regulates motor and learning behaviors by affecting dopaminergic neuron survival in mice
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Optineurin (OPTN) is an autophagy receptor that participates in the degradation of damaged mitochondria, protein aggregates, and invading pathogens. OPTN is closely related to various types of neurodegenerative diseases. However, the role of OPTN in the central nervous system is unclear. Here, we found that OPTN dysregulation in the compact part of substantia nigra (SNc) led to motor and learning deficits in animal models. Knockdown of OPTN increased total and phosphorylated α-synuclein levels which induced microglial activation and dopaminergic neuronal loss in the SNc. Overexpression of OPTN can't reverse the motor and learning phenotypes. Mechanistic analysis revealed that upregulation of OPTN increased α-synuclein phosphorylation independent of its autophagy receptor activity, which further resulted in microglial activation and dopaminergic neuronal loss similar to OPTN downregulation. Our study uncovers the crucial role of OPTN in maintaining dopaminergic neuron survival and motor and learning functions which are disrupted in PD patients.
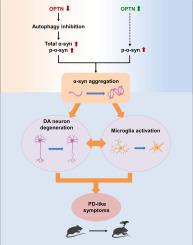
Optineurin通过影响小鼠多巴胺能神经元的存活来调节运动和学习行为。
Optineurin(OPTN)是一种自噬受体,参与降解受损线粒体、蛋白质聚集体和入侵病原体。OPTN 与各种类型的神经退行性疾病密切相关。然而,OPTN 在中枢神经系统中的作用尚不清楚。在这里,我们发现黑质(SNc)紧凑部分的 OPTN 失调会导致动物模型出现运动和学习障碍。敲除 OPTN 会增加总α-突触核蛋白和磷酸化α-突触核蛋白的水平,从而诱导小胶质细胞活化和黑质核团中多巴胺能神经元的缺失。过表达OPTN不能逆转运动和学习表型。机理分析表明,OPTN的上调增加了α-突触核蛋白磷酸化,这与其自噬受体活性无关,而自噬受体活性进一步导致小胶质细胞活化和多巴胺能神经元缺失,这与OPTN下调的结果类似。我们的研究揭示了OPTN在维持多巴胺能神经元存活以及运动和学习功能方面的关键作用,而这些功能在帕金森病患者中受到破坏。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


