Inhibition of TGF-β signaling in bone marrow endothelial cells promotes hematopoietic recovery in acute myeloid leukemia patients
IF 9.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Although it is an effective treatment for acute myeloid leukemia (AML), chemotherapy leads to myelosuppression and poor hematopoietic reconstruction. Hematopoiesis is regulated by bone marrow (BM) endothelial cells (ECs), and BM ECs are dysfunctional in acute leukemia patients with poor hematopoietic reconstitution after allogenic hematopoietic stem cell transplantation. Thus, it is crucial to explore the underlying mechanism of EC impairment and establish strategies for targeted therapy. TGF-β signaling was found to be upregulated in ECs from AML patients in complete remission (CR ECs) and led to CR EC damage. Administration of a TGF-β inhibitor rescued the dysfunction of ECs caused by TGF-β1 expression in vitro, especially their hematopoiesis-supporting ability. Moreover, inhibition of TGF-β expression repaired the BM EC damage triggered by chemotherapy in both AML patients in vitro and in an AML-CR murine model, and restored normal hematopoiesis without promoting AML progression. Mechanistically, our data reveal alterations in the transcriptomic pattern of damaged BM ECs, accompanied by the overexpression of downstream molecules TGF-βR1, pSmad2/3, and functional genes related to adhesion, angiogenesis suppression and pro-apoptosis. Collectively, our findings reveal for the first time that the activation of TGF-β signaling leads to BM EC dysfunction and poor hematopoietic reconstitution. Targeting TGF-β represents a potential therapeutic strategy to promote multilineage hematopoiesis, thereby benefiting more cancer patients who suffer from myelosuppression after chemotherapy.
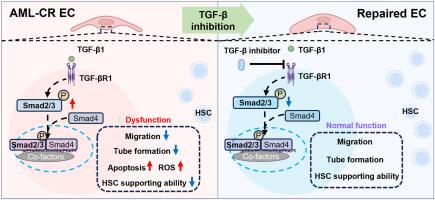
抑制骨髓内皮细胞中的 TGF-β 信号传导可促进急性髓性白血病患者的造血功能恢复。
虽然化疗是治疗急性髓性白血病(AML)的有效方法,但化疗会导致骨髓抑制和造血重建不良。造血受骨髓(BM)内皮细胞(EC)调控,急性白血病患者异基因造血干细胞移植后造血重建不良,BM EC功能失调。因此,探索EC受损的内在机制并制定靶向治疗策略至关重要。研究发现,TGF-β信号在完全缓解的急性髓细胞性白血病患者(CR ECs)的ECs中上调,并导致CR EC损伤。服用TGF-β抑制剂可挽救体外TGF-β1表达导致的EC功能障碍,尤其是其造血支持能力。此外,抑制 TGF-β 的表达还能修复急性髓细胞性白血病患者体外和急性髓细胞性白血病-慢性淋巴细胞性白血病鼠模型中化疗引发的骨髓造血干细胞损伤,并恢复正常的造血功能,而不会促进急性髓细胞性白血病的进展。从机理上讲,我们的数据揭示了受损骨髓细胞的转录组模式发生了改变,伴随着下游分子 TGF-βR1、pSmad2/3 以及与粘附、血管生成抑制和促凋亡相关的功能基因的过度表达。总之,我们的研究结果首次揭示了 TGF-β 信号的激活会导致 BM EC 功能障碍和造血重建不良。靶向 TGF-β 是一种促进多系造血的潜在治疗策略,从而使更多化疗后骨髓抑制的癌症患者受益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


