Activation of the γ-secretase/NICD-PXR/Notch pathway induces Taxol resistance in triple-negative breast cancer
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Triple-negative breast cancer (TNBC) is currently the only subtype lacking efficient targeted therapies. Taxol is the primary chemotherapeutic agent for TNBC. However, Taxol resistance often develops in the treatment of TNBC patients, which importantly contributes to high mortality and poor prognosis in TNBC patients. Recent preclinical studies have shown that the inhibition of Notch pathway by γ-secretase inhibitors can slow down the progression of TNBC. Our studies in bioinformatic analysis of breast cancer patients and TNBC/Taxol cells in vitro showed that there was high correlation between the activation of Notch pathway and Taxol resistance in TNBC. Increased γ-secretase activity (by the overexpression of catalytic core PSEN-1) significantly reduced Taxol sensitivity of TNBC cells, and enhanced biological characteristics of malignancy in vitro, and tumour growth in vivo. Mechanistically, increased γ-secretase activity led to the accumulation of NICD in the nucleus, promoting the interaction between NICD and PXR to activate PXR, which triggered the transcription of PXR downstream associated drug resistance genes. Furthermore, we showed that pharmacological inhibition of γ-secretase with γ-secretase inhibitors (Nirogacestat and DAPT) can reverse Taxol resistance in vivo and in vitro. Our results for the first time demonstrate that the activation of γ −secretase/NCD-PXR/Notch pathway is one of important mechanisms to cause Taxol resistance in TNBC, and the blockades of this pathway may represent a new therapeutic strategy for overcoming Taxol resistance in TNBC.
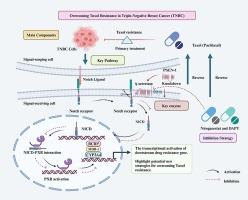
激活γ-分泌酶/NICD-PXR/Notch通路可诱导三阴性乳腺癌对紫杉醇产生抗药性。
三阴性乳腺癌(TNBC)是目前唯一缺乏高效靶向疗法的亚型。紫杉醇是治疗 TNBC 的主要化疗药物。然而,在治疗 TNBC 患者的过程中经常会出现紫杉醇耐药性,这也是 TNBC 患者死亡率高、预后差的重要原因。最近的临床前研究表明,通过γ-分泌酶抑制剂抑制Notch通路可以延缓TNBC的进展。我们对乳腺癌患者和体外 TNBC/Taxol 细胞进行的生物信息学分析表明,Notch 通路的激活与 TNBC 的 Taxol 耐药性高度相关。γ-分泌酶活性的增加(通过催化核心 PSEN-1 的过表达)显著降低了 TNBC 细胞对 Taxol 的敏感性,增强了体外恶性肿瘤的生物学特征和体内肿瘤的生长。从机理上讲,γ-分泌酶活性的增加会导致 NICD 在细胞核中的积累,促进 NICD 与 PXR 之间的相互作用,从而激活 PXR,引发 PXR 下游相关耐药基因的转录。此外,我们还发现用γ-分泌酶抑制剂(Nirogacestat和DAPT)对γ-分泌酶进行药理抑制,可以逆转体内和体外的紫杉醇耐药性。我们的研究结果首次证明,γ-分泌酶/NCD-PXR/Notch通路的激活是导致TNBC对Taxol耐药的重要机制之一,阻断该通路可能是克服TNBC对Taxol耐药的一种新的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


