The role of proton-coupled electron transfer from protein to heme in dehaloperoxidase
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2024-10-16
DOI:10.1016/j.bbapap.2024.141053
引用次数: 0
Abstract
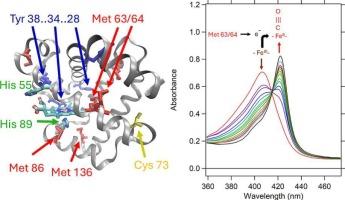
At least two of the six methionine (Met) residues in dehaloperoxidase (DHP) are shown to act as electron donors in both autoreduction and protein-heme crosslinking. Autoreduction observed in the two isozymes, DHP-A and DHP-B, is explained by the high heme reduction potential and an endogenous source of electrons from methionine (Met) or cysteine (Cys). This study provides evidence of a connection to protein-heme crosslinking that occurs when DHP is activated by H2O2 in competition with substrate oxidation and autoreduction. The autoreduction yields of DHP-A and DHP-B are comparable and both are inversely proportional to DHP concentration. Both isoenzymes show an anti-cooperative effect on autoreduction kinetics associated with protein dimerization. Despite the presence of five tyrosine (Tyr) amino acids in DHP-A and four Tyr in DHP-B, the mass spectral evidence does not support a Tyr-heme or interprotein Tyr-Tyr crosslinking event as observed in some mammalian myoglobins. LC-MS and tandem MS/MS studies revealed three amino acids that were involved in the heme-protein crosslink, Cys73, Met63 and Met64. Cys73 facilitates dimer formation in DHP-A which also appears to slow the rate of autoreduction, but is not involved in covalent protein-heme crosslinking. Based on mutational studies, Met63 and 64 are involved in both covalent heme crosslinking and autoreduction. Proton-coupled electron transfer and crosslinking by Met to the heme may serve to regulate DHP function and protect it from uncontrolled oxidative damage.
脱卤过氧化物酶中从蛋白质到血红素的质子耦合电子传递的作用。
在脱卤过氧化物酶(DHP)的六个蛋氨酸(Met)残基中,至少有两个残基在自动还原和蛋白质-血红素交联过程中充当电子供体。在 DHP-A 和 DHP-B 两种同工酶中观察到的自动还原现象可通过高血红素还原电位和来自蛋氨酸(Met)或半胱氨酸(Cys)的内源性电子源来解释。本研究提供的证据表明,当 DHP 被 H2O2 激活与底物氧化和自动还原竞争时,会发生与蛋白质血红素交联的联系。DHP-A 和 DHP-B 的自动还原产率相当,且都与 DHP 浓度成反比。这两种同工酶在与蛋白质二聚化相关的自动还原动力学上都显示出反合作效应。尽管在 DHP-A 中存在五个酪氨酸(Tyr)氨基酸,在 DHP-B 中存在四个酪氨酸,但质谱证据并不支持在某些哺乳动物肌红蛋白中观察到的酪氨酸-血红素或蛋白质间酪氨酸-酪氨酸交联事件。LC-MS 和串联 MS/MS 研究揭示了参与血红素-蛋白质交联的三个氨基酸:Cys73、Met63 和 Met64。Cys73 促进了 DHP-A 中二聚体的形成,这似乎也减缓了自动还原的速度,但并不参与共价蛋白质-血红素交联。根据突变研究,Met63 和 64 既参与共价血红素交联,也参与自动还原。质子耦合电子传递和 Met 与血红素的交联可能有助于调节 DHP 的功能,保护其免受失控的氧化损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


