EcNikA, a versatile tool in the field of artificial metalloenzymes
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
This review describes the multiple advantages of using of EcNikA, a nickel transport protein, in the design of artificial metalloenzymes as alternative catalysts for synthetic biology. The rationale behind the strategy of artificial enzyme design is discussed, with particular emphasis on de novo active site reconstitution. The impact of the protein scaffold on the artificial active site and thus the final catalytic properties is detailed, highlighting the considerable aptitude of hybrid systems to catalyze selective reactions, from alkene to thioether transformations (epoxidation, hydroxychlorination, sulfoxidation). The different catalytic approaches – from in vitro to in cristallo – are compared, revealing the considerable advantages of protein crystals in terms of stabilization and acceleration of reaction kinetics. The versatility of proteins, based on metal and ligand diversity and medium/physical conditions, are thus illustrated for oxidation catalysis.
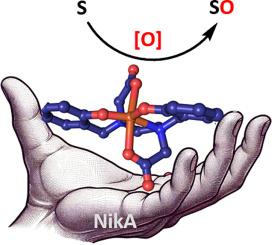
EcNikA,人工金属酶领域的多功能工具。
本综述介绍了使用镍转运蛋白 EcNikA 设计人工金属酶作为合成生物学替代催化剂的多种优势。文章讨论了人工酶设计策略的基本原理,特别强调了活性位点的重新构建。详细介绍了蛋白质支架对人工活性位点的影响以及最终的催化特性,强调了混合系统在催化从烯到硫醚转化(环氧化、羟基氯化、硫氧化)等选择性反应方面的巨大能力。比较了从体外到晶体内的不同催化方法,揭示了蛋白质晶体在稳定和加速反应动力学方面的巨大优势。基于金属和配体的多样性以及介质/物理条件,蛋白质在氧化催化方面的多功能性由此可见一斑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


