Evaluating delivery of peptide nucleic acids to Gram-negative bacteria using differently linked membrane-active peptides and their stapled analogs
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Antisense oligonucleotides have been developed as therapeutic compounds, with peptide nucleic acid (PNA) emerging as a promising nucleic acid mimic for antimicrobial applications. To be effective, PNAs must be internalized into bacterial cells, as they are not naturally absorbed. A strategy to improve PNA membrane penetration and cellular uptake involves covalently conjugating them to cell-penetrating peptides. However, these membrane-active peptides can exhibit cytotoxicity, and their efficiency as PNA carriers needs to be enhanced. Therefore, we explored new peptide–PNA conjugates and their linkers to understand how they affect PNA uptake into bacteria. We conjugated PNA to two peptides, anoplin and (KFF)3K, along with their structurally stabilized hydrocarbon-stapled derivatives, and evaluated their transport into various bacterial strains. The PNA sequence targeted bacterial mRNA encoding the essential acyl carrier protein. As linkages, we used either a non-cleavable 8-amino-2,6-dioxaoctanoyl (ethylene glycol, eg1) linker or a reducible disulfide bridge. We found that the hydrocarbon-stapled peptides did not enhance PNA delivery, despite the strong inner- and outer-membrane-penetrating capabilities of the standalone peptides. Additionally, the disulfide bridge linkage, which is cleavable in the bacterial cytoplasm, decreased the antimicrobial activity of the peptide–PNA conjugates. Notably, we identified anoplin as a new potent PNA carrier peptide, with the anoplin–eg1–PNA conjugate demonstrating antibacterial activity against E. coli and S. Typhimurium strains in the 2–4 µM range.
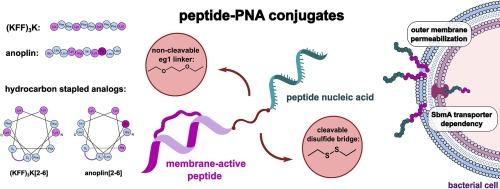
评估使用不同连接的膜活性肽及其订书钉类似物向革兰氏阴性菌输送肽核酸的情况。
反义寡核苷酸已被开发为治疗化合物,而肽核酸(PNA)则是一种很有前途的抗菌核酸模拟物。由于肽核酸不会被自然吸收,因此必须内化到细菌细胞中才能有效。改善 PNA 膜穿透性和细胞吸收的一种策略是将其与细胞穿透肽共价共轭。然而,这些膜活性肽会表现出细胞毒性,因此需要提高它们作为 PNA 载体的效率。因此,我们探索了新的多肽-PNA 共轭物及其连接体,以了解它们如何影响细菌对 PNA 的吸收。我们将 PNA 与两种肽(anoplin 和 (KFF)3K)及其结构稳定的碳氢化合物叠层衍生物共轭,并评估了它们在不同细菌菌株中的转运情况。PNA 序列以编码必需酰基载体蛋白的细菌 mRNA 为目标。作为连接,我们使用了不可裂解的 8-氨基-2,6-二氧杂辛酰(乙二醇,eg1)连接体或可还原的二硫桥。我们发现,尽管独立肽具有很强的内膜和外膜穿透能力,但碳氢化合物叠层肽并不能增强 PNA 的输送。此外,二硫桥连接(可在细菌细胞质中裂解)降低了肽-PNA 共轭物的抗菌活性。值得注意的是,我们发现anoplin是一种新的强效PNA载体肽,anoplin-eg1-PNA共轭物对大肠杆菌和鼠伤寒杆菌的抗菌活性在2-4 µM范围内。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


