Diffusion of 1O2 along the PNA backbone diminishes the efficiency of photooxidation of PNA/DNA duplexes by biphenyl photosensitizer
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
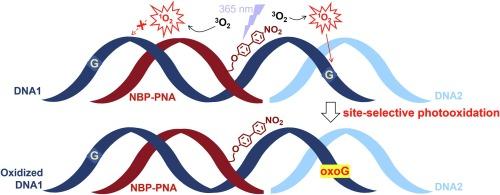
Nitrobiphenyl photosensitizer (NBP)-peptide nucleic acids (PNA) conjugates were synthesized to develop a tool for photo-knockdown of target DNAs. The presence of NBP hardly hindered duplex formation with the complementary single strand DNA as demonstrated by the comparison of Tm values and CD spectra with those for standard PNA/DNA duplexes. However, the photooxidation of guanines in NBP-PNA/DNAs was significantly less effective than those of corresponding NBP-DNA/DNA. Production of singlet oxygen (1O2) during the photooxidation was confirmed by consumption of furfuryl alcohol, a 1O2 detector. The poor photooxidation efficiency was ameliorated with 1O2 generated from an externally added NBP derivative. It was found that, when complexed with the sticky end of a double strand DNA, NBP-PNA was able to photooxidize G in the DNA/DNA duplex region, whereas G in the PNA/DNA duplex region was considerably unreactive. These results suggest that 1O2 produced from NBP-PNA tends to quench during diffusion along the PNA/DNA backbone, whereas quenching is less likely during diffusion along DNA/DNA region.
1O2 沿 PNA 主干扩散会降低联苯光敏剂对 PNA/DNA 双链体的光氧化效率。
我们合成了硝基联苯光敏剂(NBP)-肽核酸(PNA)共轭物,以开发一种光敲除目标 DNA 的工具。与标准 PNA/DNA 双链的 Tm 值和 CD 光谱相比,NBP 的存在几乎不妨碍与互补单链 DNA 形成双链。然而,NBP-PNA/DNA 中鸟嘌呤的光氧化作用明显不如相应的 NBP-DNA/DNA 的光氧化作用。光氧化过程中产生的单线态氧(1O2)通过消耗 1O2 检测器糠醇得到了证实。外部添加的 NBP 衍生物产生的 1O2 改善了光氧化效率低下的问题。研究发现,当 NBP-PNA 与双链 DNA 的粘性末端复合时,NBP-PNA 能够光氧化 DNA/DNA 双链区中的 G,而 PNA/DNA 双链区中的 G 则明显没有反应。这些结果表明,NBP-PNA 产生的 1O2 在沿 PNA/DNA 主干扩散时趋于淬灭,而在沿 DNA/DNA 区域扩散时则不太可能淬灭。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


