Novel bibenzyl compound 8Ae induces apoptosis and inhibits glycolysis by detaching hexokinase 2 from mitochondria in A549 cells
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
In this paper, we investigated the anticancer effect and the mechanism of our newly synthesized bibenzyl 8Ae against human lung cancer A549 cells. Compound 8Ae could induce apoptosis by inhibiting the glycolysis in A549 cells. Hexokinase 2 (HK2), the first key enzyme in glycolysis process, was significantly down-regulated by 8Ae. Besides, compound 8Ae induced HK2 dissociated from mitochondria to cytosol, which could be induced by inhibiting the phosphorylation of Akt. In addition, 8Ae could induce mitochondrial-mediated apoptosis, and mitochondrial membrane potential (MMP) was decreased. After 8Ae treatment, the Bax/Bcl-2 ratio was increased and cytochrome c (Cyt c) was release from mitochondria to cytosol. Molecular docking indicated that 8Ae have an interaction with HK2 by extending into acitve pockets of the protein to form stable hydrogen bonds. Additionally, 8Ae had significantly improved pharmacokinetic properties through the prediction, comparison, and analysis of the ADMET properties of 8Ae and moscatilin (MST). Taken together, 8Ae might inhibit glycolysis by stimulating the shedding of HK2 from mitochondria and promoting mitochondria-regulated apoptosis to inhibit the proliferation of A549 cells. This article provides a research basis for bibenzyl compounds as new small molecule drugs for lung cancer.
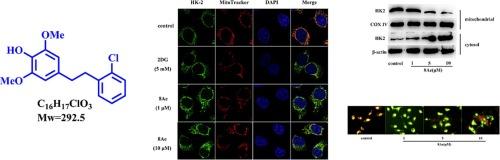
新型双苄化合物 8Ae 可通过从线粒体分离己糖激酶 2 来诱导 A549 细胞凋亡并抑制糖酵解。
本文研究了新合成的联苄 8Ae 对人类肺癌 A549 细胞的抗癌作用及其机制。化合物 8Ae 可通过抑制 A549 细胞的糖酵解作用诱导细胞凋亡。糖酵解过程中的第一个关键酶六磷酸酶2(HK2)被8Ae显著下调,而且化合物8Ae能诱导HK2从线粒体分离到细胞质,这可能是通过抑制Akt的磷酸化而诱导的。此外,8Ae 还能诱导线粒体介导的细胞凋亡,线粒体膜电位(MMP)降低。经 8Ae 处理后,Bax/Bcl-2 比率升高,细胞色素 c(Cyt c)从线粒体释放到细胞膜。分子对接表明,8Ae 与 HK2 有相互作用,能延伸到蛋白质的活性袋中形成稳定的氢键。此外,通过预测、比较和分析 8Ae 和莫斯卡替林(MST)的 ADMET 特性,8Ae 的药代动力学特性得到了显著改善。综上所述,8Ae可能通过刺激线粒体中HK2的脱落和促进线粒体调控的细胞凋亡来抑制糖酵解,从而抑制A549细胞的增殖。本文为双苄基化合物作为治疗肺癌的小分子新药提供了研究基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


