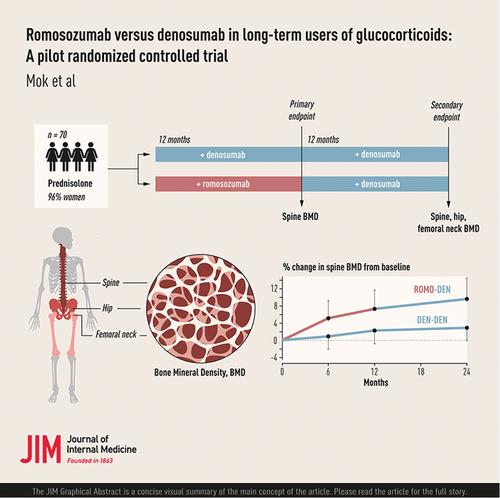
Romosozumab versus denosumab in long-term users of glucocorticoids: A pilot randomized controlled trial
Abstract
Objective
To compare the efficacy of romosozumab (ROMO) and denosumab (DEN) in prevalent long-term glucocorticoid (GC) users.
Methods
Adult patients receiving oral prednisolone (≥5 mg/day) with high risk of fracture were randomized to receive subcutaneous ROMO (210 mg monthly) or DEN (60 mg 6-monthly) for 12 months, followed by DEN for two more doses. The primary end point was the change in spine bone mineral density (BMD) from Months 0 to 12. Secondary end points included changes in BMD of the spine/hip/femoral neck and bone turnover markers at various time points and adverse events.
Results
Seventy patients (age 62.6 ± 9.1 years; 96% women; median prednisolone dose 5.0 mg/day; duration of therapy 10.7 ± 7.4 years) were enrolled, and 63 completed the study. At Month 12, the spine BMD increased significantly in both ROMO (+7.3% ± 4.5%; p < 0.001) and DEN (+2.3% ± 3.1%; p < 0.001) groups. The absolute spine BMD gain from Months 0 to 12 was significantly greater in ROMO-treated patients (p < 0.001). Although the total hip BMD at Month 12 also increased significantly in the ROMO (+1.6% ± 3.3%; p = 0.01) and DEN groups (+1.6% ± 2.6%; p = 0.003), the absolute BMD gain was not significantly different between the groups. At Month 24, the spine BMD continued to increase in both the ROMO (+9.7% ± 4.8%; p < 0.001) and DEN group (+3.0% ± 3.0%; p < 0.001) compared to baseline, and the absolute BMD gain remained significantly greater in ROMO-treated patients. The total hip BMD continued to increase in both groups (ROMO +2.9% ± 3.7%; p < 0.001; DEN +2.2% ± 3.4%; p = 0.001), but the changes from baseline were similar. Injection site reaction was more frequently reported in ROMO-treated patients.
Conclusion
ROMO was superior to DEN in raising the spine BMD at Month 12 in chronic GC users. After switching to DEN, ROMO-treated patients continued to gain spine BMD to a greater extent than DEN until Month 24.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


