Palladium-Catalyzed Dearomative Heck/C(sp2)–H Activation/Decarboxylative Cyclization of C2-Tethered Indoles
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Until now, palladium-catalyzed dearomative Heck reactions of indoles were largely limited to β-H elimination and nucleophilic capture of the transient alkyl-Pd(II) species. Herein, we disclose a novel palladium-catalyzed dearomative Heck/C(sp2)–H activation/decarboxylative cyclization of C2-tethered indoles. In this protocol, the alkyl-Pd(II) species formed by dearomatization of C2-tethered indoles is not terminated by common β-H elimination or nucleophilic capture, but rather generates C,C-palladacycle via C–H activation. The latter is intercepted by α-bromoacrylic acids to produce pentacyclic and heptacyclic fused indolines.
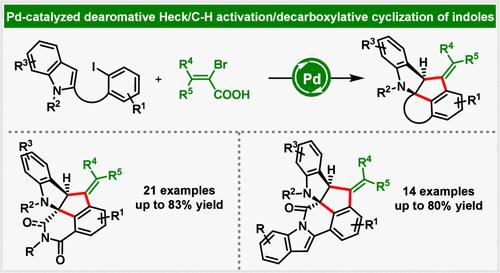
钯催化的 C2 系吲哚的 Dearomative Heck/C(sp2)-H活化/脱羧环化反应
迄今为止,钯催化的吲哚脱芳香 Heck 反应主要局限于 β-H 消去和亲核捕捉瞬时烷基钯(II)物种。在此,我们公开了一种新型的钯催化 C2 系吲哚的脱芳香 Heck/C(sp2)-H活化/脱羧环化反应。在该方案中,C2-系链吲哚脱芳香化形成的烷基-钯(II)物种不是通过普通的β-H消除或亲核捕获来终止,而是通过C-H活化生成C,C-帕拉二环。后者被 α-溴丙烯酸截取,生成五环和七环融合吲哚啉。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


