Cascade CO2 Insertion in Carbanion Ionic Liquids Driven by Structure Rearrangement
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The CO2 chemisorption in state-of-the-art sorbents based on oxide/hydroxide/amine moieties is driven by strong chemical bonding formation in the carbonate/bicarbonate/carbamate products, which in turn leads to high energy input in sorbent regeneration. In addition, the CO2 uptake capacity was limited by the active sites’ utilization efficiency, with each active site incorporating one CO2 molecule or less. In this work, a new concept and generation of sorbent was developed to achieve cascade insertion of multiple CO2 molecules by leveraging structure rearrangement as the driving force, leading to in situ generation of extra CO2-binding sites and significantly reduced energy input for CO2 release. The designed ionic liquids (ILs) containing carbanions with conjugated and asymmetric structure, deprotonated (methylsulfonyl)acetonitrile ([MSA]) anion, allowed the cascade insertion of two CO2 molecules via consecutive C–C and O–C bond formations. The proton transfer and structure rearrangement of the carboxylic acid intermediates played critical roles in stabilizing the first integrated CO2 and generating extra electron-rich oxygen sites for the insertion of the second CO2. The structure variation and reaction pathway were confirmed by operando spectroscopy, magnetic resonance spectroscopy (NMR), mass spectroscopy, and computational chemistry. The energy input in sorbent regeneration could be further reduced by harnessing the phase-changing behavior of the carbanion salts in ether solutions upon reacting with CO2, avoiding the energy consumption in heating the solvent. The fundamental insights obtained herein provide a promising approach to greatly improve the CO2 sorption performance via sophisticated molecular-scale structural engineering of the sorbents.
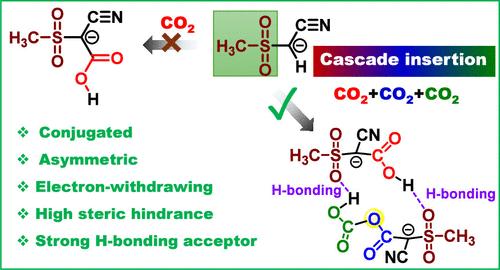
由结构重排驱动的卡巴离子液体中的级联二氧化碳插入效应
基于氧化物/氢氧化物/胺分子的最先进吸附剂对二氧化碳的化学吸附是由碳酸盐/碳酸氢盐/氨基甲酸酯产物中形成的强化学键驱动的,这反过来又导致吸附剂再生过程中的高能量输入。此外,二氧化碳吸收能力还受到活性位点利用效率的限制,每个活性位点只能吸收一个或更少的二氧化碳分子。在这项工作中,我们开发了一种新概念和新一代吸附剂,利用结构重排作为驱动力,实现多个二氧化碳分子的级联插入,从而在原位生成额外的二氧化碳结合位点,并显著减少二氧化碳释放的能量输入。所设计的离子液体(ILs)含有具有共轭和不对称结构的碳离子、去质子化(甲基磺酰基)乙腈([MSA])阴离子,可通过连续的 C-C 和 O-C 键形成实现两个二氧化碳分子的级联插入。羧酸中间体的质子转移和结构重排在稳定第一个整合的二氧化碳和为第二个二氧化碳的插入产生额外的富电子氧位方面发挥了关键作用。操作光谱、磁共振光谱(NMR)、质谱和计算化学证实了结构变化和反应途径。通过利用乙醚溶液中的碳阴离子盐在与二氧化碳反应时的相变行为,可进一步减少吸附剂再生过程中的能量输入,从而避免加热溶剂时的能量消耗。本文所获得的基本见解为通过对吸附剂进行复杂的分子尺度结构工程设计来大大提高二氧化碳吸附性能提供了一种可行的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


