Kinetics and Mechanism of PPh3/Ni-Catalyzed, Zn-Mediated, Aryl Chloride Homocoupling: Antagonistic Effects of ZnCl2/Cl–
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
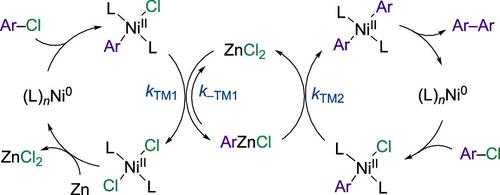
The Ni/PPh3-catalyzed homocoupling of aryl chlorides in DMF using Zn as the stochiometric reducing agent is one of a general class of Ni-catalyzed processes, where the mechanism has been a matter of long-standing debate. This study re-evaluates prior conclusions and insights. NMR spectroscopy is used to identify [(PPh3)2NiII(Ar)Cl] as a key intermediate and to explore the indirect roles of using Zn as the reductant. The [ZnCl2] coproduct is responsible for several features, including a sequential transmetalation pathway involving [ArZnCl]. [ZnCl2] also abstracts halide from [(PPh3)2NiCl2] to generate [NiIICl(DMF)5]+[ZnCl3(DMF)]−, and in doing so, affects the NiII + Ni0 ↔ 2 NiI speciation. [ZnCl2] thus acts as an accelerator and inhibitor, resulting in mildly sigmoidal reaction profiles. When the [ZnCl2] concentration becomes too high or the phosphine ligand concentration too low, catalysis stalls. Turnover is restored by the addition of further phosphine ligand, or chloride ion. In the presence of an exogenous chloride ion, turnover is rapid, again proceeding via [(PPh3)2NiII(Ar)Cl] but via dinuclear metathesis. The generation of [ZnCl3(DMF)]− results in mutually antagonistic effects between [ZnCl2] and [Cl]− such that turnover proceeds via one mechanism or the other, depending on which species is in excess. The intermediacy of [ArZnCl] suggests a solution to the long-standing anomaly that many other reductants were found to be much less effective than Zn in inducing turnover of Ni/PPh3 catalyzed aryl chloride homocoupling in DMF. The use of DMAc as a solvent in place of DMF inhibits stalling through the steric inhibition of mixed metalate generation.
锌介导的 PPh3/Ni- 催化芳基氯均偶联反应的动力学和机理:ZnCl2/Cl- 的拮抗作用
镍/PPh3 催化的芳基氯化物在 DMF 中的均偶联反应是镍催化的一类常见反应,其机理长期以来一直存在争议。本研究重新评估了之前的结论和见解。利用核磁共振光谱确定[(PPh3)2NiII(Ar)Cl]为关键中间体,并探索使用 Zn 作为还原剂的间接作用。[ZnCl2]副产物具有多种特征,包括涉及[ArZnCl]的连续转金属途径。[ZnCl2]还能从[(PPh3)2NiCl2]中抽取卤化物,生成[NiIICl(DMF)5]+[ZnCl3(DMF)]-,从而影响 NiII + Ni0 ↔ 2 NiI 的分型。因此,[ZnCl2]既是促进剂,又是抑制剂,导致反应曲线呈轻微的西格玛曲线。当[ZnCl2]浓度过高或膦配体浓度过低时,催化作用就会停止。再加入膦配体或氯离子就能恢复周转。在存在外源氯离子的情况下,翻转速度很快,同样通过[(PPh3)2NiII(Ar)Cl]进行,但通过的是双核偏析。ZnCl3(DMF)]- 的生成会导致[ZnCl2]和[Cl]-之间产生相互拮抗的作用,因此翻转会通过一种机制或另一种机制进行,具体取决于哪种物质过量。ArZnCl]的中间性为长期存在的异常现象提供了一种解决方案,即在 DMF 中,许多其他还原剂在诱导 Ni/PPh3 催化的芳基氯均偶合反应的翻转方面远不如锌有效。使用 DMAc 代替 DMF 作为溶剂,可以通过立体抑制混合金属酸盐的生成来抑制反应停滞。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


