Protein Recruitment to Dynamic DNA-RNA Host Condensates
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We describe the design and characterization of artificial nucleic acid condensates that are engineered to recruit and locally concentrate proteins of interest in vitro. These condensates emerge from the programmed interactions of nanostructured motifs assembling from three DNA strands and one RNA strand that can include an aptamer domain for the recruitment of a target protein. Because condensates are designed to form regardless of the presence of target protein, they function as “host” compartments. As a model protein, we consider Streptavidin (SA) due to its widespread use in binding assays. In addition to demonstrating protein recruitment, we describe two approaches to control the onset of condensation and protein recruitment. The first approach uses UV irradiation, a physical stimulus that bypasses the need for exchanging molecular inputs and is particularly convenient to control condensation in emulsion droplets. The second approach uses RNA transcription, a ubiquitous biochemical reaction that is central to the development of the next generation of living materials. We then show that the combination of RNA transcription and degradation leads to an autonomous dissipative system in which host condensates and protein recruitment occur transiently and that the host condensate size as well as the time scale of the transition can be controlled by the level of RNA-degrading enzyme. We conclude by demonstrating that biotinylated beads can be recruited to SA-host condensates, which may therefore find immediate use for the physical separation of a variety of biotin-tagged components.
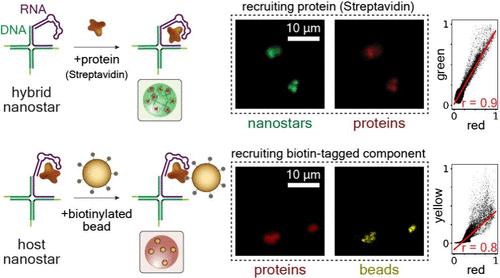
动态 DNA-RNA 宿主凝聚体的蛋白质招募
我们介绍了人工核酸凝聚体的设计和特性,这些凝聚体经过设计可在体外招募和局部浓缩感兴趣的蛋白质。这些凝聚体是由三条 DNA 链和一条 RNA 链组装而成的纳米结构图案的程序化相互作用产生的,这些图案可以包括一个用于招募目标蛋白质的适配体结构域。由于聚合体的设计目的是在目标蛋白质不存在的情况下形成,因此它们具有 "宿主 "区室的功能。由于链霉亲和素(Streptavidin,SA)在结合试验中的广泛应用,我们将其作为模型蛋白。除了展示蛋白质招募外,我们还介绍了两种控制凝结开始和蛋白质招募的方法。第一种方法使用紫外线照射,这是一种物理刺激,无需交换分子输入,特别方便控制乳液液滴的凝结。第二种方法使用 RNA 转录,这是一种无处不在的生化反应,对下一代生命材料的开发至关重要。我们随后证明,RNA 转录和降解的结合会产生一个自主耗散系统,在这个系统中,宿主凝结物和蛋白质招募会短暂发生,宿主凝结物的大小以及转变的时间尺度可由 RNA 降解酶的水平控制。最后,我们证明了生物素化珠子可以被吸附到 SA-宿主凝聚物上,因此可以立即用于各种生物素标记成分的物理分离。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


