Pharmacophore-Based Modeling, Synthesis, and Biological Evaluation of Novel Quinazoline/Quinoline Derivatives: Discovery of EGFR Inhibitors with Low Nanomolar Activity
IF 2.9
4区 工程技术
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
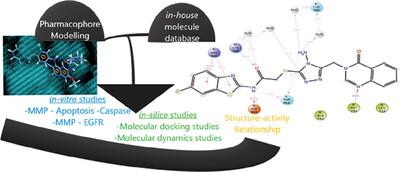
The main aim of this study is to obtain novel molecules that are more selective on cancer cells compared to healthy cells. For this purpose, four hit molecules are identified using 11 new pharmacophore hypotheses followed by scanning the in-house database. Then, based on those hit molecules, the synthesis and analysis of four different series (three quinazolines and one quinoline series) are carried out, and their anticancer activity is investigated. Finally, by using molecular docking and dynamics simulation methods, binding mode and structure–activity relationship are examined. Among the quinazolin-4(3H)-one derivatives, those containing halogen atom are found to be potentially effective, while the best epidermal growth factor receptor (EGFR) inhibition and apoptosis induction are displayed by compounds containing 4-amino-1,2,4-triazole moiety. Notably, four compounds (4h, 8d, 8l, and 8m) show EGFR inhibition activity at 5.298 ± 0.164, 5.46 ± 0.221, 2.670 ± 0.124, and 2.191 ± 0.908 × 10−9 m, their inhibitory activity is similar to or stronger than gefitinib (IC50: 4.169 ± 0.156 × 10−9 m). In addition, EGFR inhibitor concentration of 4g, 8e, and 8o is determined as 27588 ± 6.945, 52.41 ± 2.312, and 33657 ± 8.512 × 10−9 m. These findings indicate that generated pharmacophore hypotheses successfully determine new EGFR inhibitors. In conclusion, four novel compounds, more active than gefitinib with fewer side effects, are reached, and the structure–activity relationships are clarified.
基于药理的新型喹唑啉/喹啉衍生物的建模、合成和生物学评价:发现具有低纳摩尔活性的表皮生长因子受体抑制剂
这项研究的主要目的是获得对癌细胞比对健康细胞更具选择性的新型分子。为此,通过扫描内部数据库,利用 11 个新的药效假说确定了 4 个命中分子。然后,在这些命中分子的基础上,合成和分析了四个不同的系列(三个喹唑啉系列和一个喹啉系列),并研究了它们的抗癌活性。最后,利用分子对接和动力学模拟方法,研究了它们的结合模式和结构-活性关系。在喹唑啉-4(3H)-酮衍生物中,发现含有卤素原子的衍生物具有潜在的抗癌活性,而含有 4-氨基-1,2,4-三唑分子的化合物对表皮生长因子受体(EGFR)的抑制和凋亡诱导效果最好。值得注意的是,四个化合物(4h、8d、8l 和 8m)的表皮生长因子受体抑制活性分别为 5.298 ± 0.164、5.46 ± 0.221、2.670 ± 0.124 和 2.191 ± 0.908 × 10-9 m,其抑制活性与吉非替尼(IC50:4.169 ± 0.156 × 10-9 m)相似或更强。此外,4g、8e 和 8o 的表皮生长因子受体抑制浓度分别为 27588 ± 6.945、52.41 ± 2.312 和 33657 ± 8.512 × 10-9 m。总之,我们找到了比吉非替尼活性更强、副作用更小的四种新型化合物,并阐明了其结构-活性关系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Theory and Simulations
Multidisciplinary-Multidisciplinary
CiteScore
5.50
自引率
3.00%
发文量
221
期刊介绍:
Advanced Theory and Simulations is an interdisciplinary, international, English-language journal that publishes high-quality scientific results focusing on the development and application of theoretical methods, modeling and simulation approaches in all natural science and medicine areas, including:
materials, chemistry, condensed matter physics
engineering, energy
life science, biology, medicine
atmospheric/environmental science, climate science
planetary science, astronomy, cosmology
method development, numerical methods, statistics
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


