Characterization of complexation of uracil with peptides through its volumetric properties in buffer saline solution at different temperatures
IF 2.2
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Densities of mixtures of uracil (Ura) with glycyl-glycine (GlyGly) and glycyl-phenylalanine (GlyPhe) in a buffered saline (pH 7.4) were measured at T=(288.15, 293.15, 298.15, 303.15, 308.15 and 313.15) K using the density meter DMA 5000 M (Anton Paar). The apparent molar volumes, limiting apparent molar volumes at infinite dilution and their derivatives with respect to temperature have been evaluated. Interactions of uracil (Ura) with glycylpeptides (GlyGly, GlyPhe, GlyTyr, GlyGlu) were studied with using review volumetric properties obtained early for Ura in GlyTyr and GlyGlu buffered solutions. The effect of concentration, ionic state and hydrophobicity of reagents on the Ura volume properties were discussed. The results indicate the complex formation between Ura and glycylpeptides with 1:1 stoichiometry. It was shown that the complexation of uracil with the glycylpeptides is accompanied by the positive changes of molar volume of Ura. The positive values of Hepler parameter indicate an increase in the ordering of the solvent in the environment of uracil with the addition of peptides. Uracil acts as a structure maker and its structure-making ability increases in series: Ura < GlyPhe < GlyGly < GlyGlu < GlyTyr.
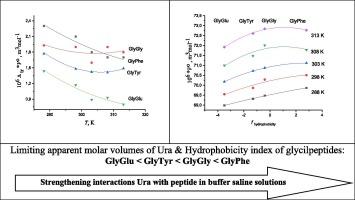
通过不同温度下缓冲盐溶液中尿嘧啶的体积特性分析尿嘧啶与肽的复合物特性
使用密度计 DMA 5000 M(Anton Paar)测量了尿嘧啶(Ura)与甘氨酰-甘氨酸(GlyGly)和甘氨酰-苯丙氨酸(GlyPhe)在缓冲盐水(pH 7.4)中的混合物密度,温度分别为(288.15、293.15、298.15、303.15、308.15 和 313.15)K。评估了表观摩尔体积、无限稀释时的极限表观摩尔体积及其随温度变化的导数。研究了尿嘧啶(Ura)与糖肽(GlyGly、GlyPhe、GlyTyr、GlyGlu)的相互作用,并回顾了早期获得的 Ura 在 GlyTyr 和 GlyGlu 缓冲溶液中的体积特性。讨论了试剂的浓度、离子状态和疏水性对 Ura 体积特性的影响。结果表明,Ura 与糖肽之间形成了 1:1 的复合物。结果表明,尿嘧啶与糖肽的络合伴随着 Ura 摩尔体积的正向变化。赫普勒参数的正值表明,随着肽的加入,尿嘧啶环境中溶剂的有序性增强。尿嘧啶是一种结构制造者,其结构制造能力随系列的增加而增强:Ura < GlyPhe < GlyGly < GlyGlu < GlyTyr。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Thermodynamics
工程技术-热力学
CiteScore
5.60
自引率
15.40%
发文量
199
审稿时长
79 days
期刊介绍:
The Journal of Chemical Thermodynamics exists primarily for dissemination of significant new knowledge in experimental equilibrium thermodynamics and transport properties of chemical systems. The defining attributes of The Journal are the quality and relevance of the papers published.
The Journal publishes work relating to gases, liquids, solids, polymers, mixtures, solutions and interfaces. Studies on systems with variability, such as biological or bio-based materials, gas hydrates, among others, will also be considered provided these are well characterized and reproducible where possible. Experimental methods should be described in sufficient detail to allow critical assessment of the accuracy claimed.
Authors are encouraged to provide physical or chemical interpretations of the results. Articles can contain modelling sections providing representations of data or molecular insights into the properties or transformations studied. Theoretical papers on chemical thermodynamics using molecular theory or modelling are also considered.
The Journal welcomes review articles in the field of chemical thermodynamics but prospective authors should first consult one of the Editors concerning the suitability of the proposed review.
Contributions of a routine nature or reporting on uncharacterised materials are not accepted.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


