Deeply insight into the inhibition mechanism of SO2 on mercury oxidation over Mn/CNT: A DFT study
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Mn/CNT is a kind of low temperature catalyst, which can effectively remove elemental mercury from flue gas at low temperature, but its mercury removal ability was significantly inhibited by SO2. In this work, the inhibition mechanism of SO2 was investigated by first-principle calculation based on density functional theory. Three different surfaces of Mn/CNT were constructed: MnO/CNT, MnO2/CNT and Mn2O3/CNT, and the adsorption of Hg0 and SO2 on these three surfaces were studied. It suggests that adsorption energy of Hg0 on MnO/CNT is the highest, which is −2.42 eV. Combined with the electron partial density of states (PDOS) after adsorption, SO2 can compete with Hg0 for the same Mn active site on the catalyst surface during the adsorption process, and the adsorption capacity of Hg0 is weaker than that of SO2. Oxidation path of Hg0 to HgO on the surface of Mn/CNT indicates that the generation of HgO needs to cross at least two energy barriers, with energies of 0.552 eV and 1.25 eV, respectively. However, when Hg0 was oxidized to HgO and adsorbed on the surface of Mn/CNT, SO2 could reduce it to Hg0 again, generating SO3 to block elemental mercury oxidation.
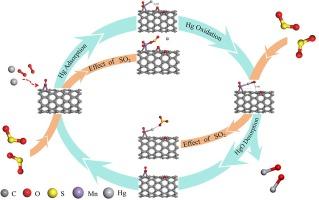
深入探讨二氧化硫对 Mn/CNT 氧化汞的抑制机理:DFT 研究
Mn/CNT 是一种低温催化剂,可在低温条件下有效去除烟气中的元素汞,但其脱汞能力受到 SO2 的明显抑制。本文基于密度泛函理论,通过第一性原理计算研究了 SO2 的抑制机理。构建了三种不同的 Mn/CNT 表面:研究了这三种表面对 Hg0 和 SO2 的吸附情况。结果表明,Hg0 在 MnO/CNT 上的吸附能最高,为 -2.42 eV。结合吸附后的电子部分态密度(PDOS),在吸附过程中,SO2 可与 Hg0 竞争催化剂表面上相同的 Mn 活性位点,且 Hg0 的吸附能力弱于 SO2。Hg0 在 Mn/CNT 表面氧化成 HgO 的路径表明,HgO 的生成至少需要跨越两个能垒,能量分别为 0.552 eV 和 1.25 eV。然而,当 Hg0 被氧化成 HgO 并吸附在 Mn/CNT 表面时,SO2 可以将其再次还原成 Hg0,生成 SO3,从而阻止元素汞的氧化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


