Magnesiation and calciation of CH acidic N-alkyl imidazoles
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
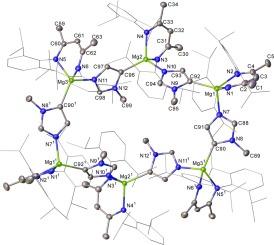
The reactivity of β-diketiminato alkylmagnesium, hydridomagnesium and hydridocalcium bases toward 1-methylimidazole, 1-tert-butylimidazole and 1-methylbenzimidazole enables the C-magnesiation and calciation of this family of N-heterocyclic molecules. Although 1-methylimidazole, 1-tert-butylimidazole, and 1-methylbenzimidazole are deprotonated at their C-2 positions by both the magnesium alkyl and hydride derivatives, these reagents can also leverage a level of regiodivergent kinetic discrimination and resultant C-4 metallation. In contrast to the straightforward C-2-deprotonation of 1-methylbenzimidazole provided by the magnesium hydride, a rapid cascade of apparent deprotonation, imidazole ring opening and twofold C![]() C coupling was induced by the analogous calcium reagent. This process provides a tricalcium species comprising an unprecedented trianionic unit, which may be considered as a trimethylenemethane dianion isostere equipped with a pendent amide donor. While attempted boron functionalisation of the imidazolyl anions of the magnesium derivatives of 1-methylimidazole and 1-methylbenzimidazole with pinacolborane (HBpin) was unsuccessful, the 1-tert-butylimidazolyl analogue provided the C-2-borylated product irrespective of the position of initial C-2 or C-4 deprotonation. Although the calcium congeners resulting from 1-methylimidazole and 1-tert-butylimidazole were deduced to form similarly borylated products, study of their onward reactivity was prevented by their instability toward Schlenk-type redistribution in solution.
C coupling was induced by the analogous calcium reagent. This process provides a tricalcium species comprising an unprecedented trianionic unit, which may be considered as a trimethylenemethane dianion isostere equipped with a pendent amide donor. While attempted boron functionalisation of the imidazolyl anions of the magnesium derivatives of 1-methylimidazole and 1-methylbenzimidazole with pinacolborane (HBpin) was unsuccessful, the 1-tert-butylimidazolyl analogue provided the C-2-borylated product irrespective of the position of initial C-2 or C-4 deprotonation. Although the calcium congeners resulting from 1-methylimidazole and 1-tert-butylimidazole were deduced to form similarly borylated products, study of their onward reactivity was prevented by their instability toward Schlenk-type redistribution in solution.
镁化和煅烧 CH 酸性 N-烷基咪唑
β-二酮亚甲基烷基镁、氢化镁和氢化钙碱对 1-甲基咪唑、1-叔丁基咪唑和 1-甲基苯并咪唑的反应性使这一系列 N-杂环分子的 C-镁化和钙化成为可能。虽然 1-甲基咪唑、1-叔丁基咪唑和 1-甲基苯并咪唑的 C-2 位都会被镁的烷基和氢化物衍生物去质子化,但这些试剂也可以利用一定程度的区域差异动力学鉴别,从而实现 C-4 金属化。与氢化镁提供的 1-甲基苯并咪唑直接的 C-2 去质子化不同,类似的钙试剂诱导了明显的去质子化、咪唑开环和两倍 CC 偶联的快速级联反应。这一过程提供了一种由前所未有的三阴离子单元组成的三钙试剂,可将其视为配备了一个悬垂酰胺供体的三亚甲基二元等效物。用频哪醇硼烷(HBpin)对 1-甲基咪唑和 1-甲基苯并咪唑的镁衍生物咪唑阴离子进行硼官能化的尝试并不成功,而 1-叔丁基咪唑类似物则提供了 C-2 硼酰化产物,与最初的 C-2 或 C-4 去质子化位置无关。虽然推断出 1-甲基咪唑和 1-叔丁基咪唑产生的钙同系物也会形成类似的硼烷基化产物,但由于它们在溶液中不稳定的 Schlenk 型再分布,阻碍了对其后续反应性的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


