Adverse outcome pathway networks as the basis for the development of new approach methodologies: Liver toxicity as a case study
IF 4.6
引用次数: 0
Abstract
The fields of toxicology and risk assessment are witnessing a paradigm shift moving away from animal testing towards the use of nonanimal and human-based new approach methodologies (NAMs). NAMs are fed by mechanistic information captured in adverse outcome pathway (AOP) networks, which are being developed and optimized at high pace. The present paper demonstrates this (r)evolution for the case of liver toxicity induced by pharmaceutical drugs. NAMs, in casu designed to predict hepatotoxicity, are composed of an in vitro system linked with a suite of assays mechanistically anchored in relevant AOP networks. These NAMs allow tiered testing at the transcriptional, translational and functionality level at high predictive capacity. Although promising, however, several challenges in NAM development still need to be tackled and are discussed in this paper.
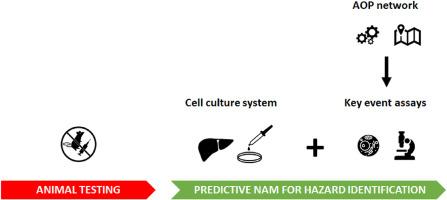
将不良后果路径网络作为开发新方法的基础:以肝脏毒性为例
毒理学和风险评估领域正在发生范式转变,从动物试验转向使用非动物和以人为基础的新方法(NAMs)。新方法由不良后果途径(AOP)网络中捕获的机理信息提供支持,这些网络正在高速发展和优化。本文以药物引起的肝脏毒性为例,展示了这种(再)进化。NAMs 是专为预测肝毒性而设计的,它由一个体外系统和一套相关 AOP 网络中机理锚定的检测方法组成。这些 NAM 可以在转录、转化和功能层面进行分层测试,具有很高的预测能力。尽管前景广阔,但在 NAM 开发过程中仍有一些挑战需要解决,本文将对此进行讨论。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current opinion in toxicology
Toxicology, Biochemistry
CiteScore
8.50
自引率
0.00%
发文量
0
审稿时长
64 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


