Opposing regulation of the STING pathway in hepatic stellate cells by NBR1 and p62 determines the progression of hepatocellular carcinoma
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Hepatocellular carcinoma (HCC) emerges from chronic inflammation, to which activation of hepatic stellate cells (HSCs) contributes by shaping a pro-tumorigenic microenvironment. Key to this process is p62, whose inactivation leads to enhanced hepatocarcinogenesis. Here, we show that p62 activates the interferon (IFN) cascade by promoting STING ubiquitination by tripartite motif protein 32 (TRIM32) in HSCs. p62, binding neighbor of BRCA1 gene 1 (NBR1) and STING, triggers the IFN cascade by displacing NBR1, which normally prevents the interaction of TRIM32 with STING and its subsequent activation. Furthermore, NBR1 also antagonizes STING by promoting its trafficking to the endosome-lysosomal compartment for degradation independent of autophagy. Of functional relevance, NBR1 deletion completely reverts the tumor-promoting function of p62-deficient HSCs by rescuing the inhibited STING-IFN pathway, thus enhancing anti-tumor responses mediated by CD8+ T cells. Therefore, NBR1 emerges as a synthetic vulnerability of p62 deficiency in HSCs by promoting the STING/IFN pathway, which boosts anti-tumor CD8+ T cell responses to restrain HCC progression.
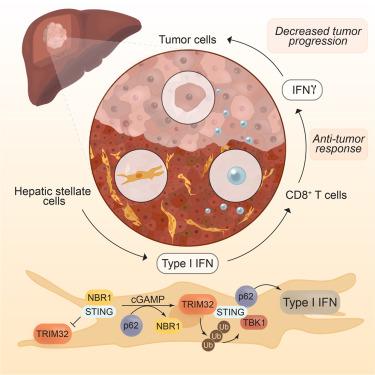
NBR1 和 p62 对肝星状细胞 STING 通路的对立调控决定了肝细胞癌的发展进程
肝细胞癌(HCC)源于慢性炎症,而肝星状细胞(HSCs)的活化则有助于形成有利于肿瘤的微环境。这一过程的关键是 p62,它的失活会导致肝癌发生的增强。p62 是 BRCA1 基因 1(NBR1)和 STING 的结合邻居,通过取代 NBR1 触发 IFN 级联,而 NBR1 通常会阻止 TRIM32 与 STING 的相互作用及其随后的激活。此外,NBR1 还能拮抗 STING,因为它能促进 STING 被转运到内膜体-溶酶体区进行降解,而不依赖于自噬。与功能相关的是,NBR1的缺失可通过挽救被抑制的STING-IFN通路,完全恢复p62缺陷造血干细胞的肿瘤促进功能,从而增强CD8+ T细胞介导的抗肿瘤反应。因此,NBR1通过促进STING/IFN通路,增强了CD8+ T细胞的抗肿瘤反应,从而抑制了HCC的进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


