Synthesis of kanamycin-azole hybrids and investigation of their antifungal activities
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The World Health Organization (WHO) recognizes Candida albicans and Cryptococcus neoformans as the critical priority fungal pathogens for which therapeutic solutions are needed. Azole-based antifungal agents, including triazoles, diazoles, and thiazoles, are widely used in the treatments for fungal infections. In light of past successes in the transformation of antibacterial kanamycin into antifungal derivatives via chemical modifications, a new library of kanamycin-azole hybrids was synthesized and tested against a panel of azole-resistant and susceptible Candida and Cryptococcus strains. Structure activity relationship (SAR) studies revealed pivotal roles for antifungal activity of the azole ring (imidazole vs triazole) and halogen substituents on the benzene ring (F vs Cl). Most notably, hybrids 13, 14 and 15 were active against resistant C. albicans, C. tropicalis and C. neoformans strains and non-toxic towards mammalian cells. Mode of action investigations using fluorogenic dyes, (SYTOXTM) showed the fungal active compounds could permeabilize fungal membranes even at ¼ MICs. These findings reveal novel azole-based antifungals that could offer new therapeutic options for candidiasis and cryptococcosis.
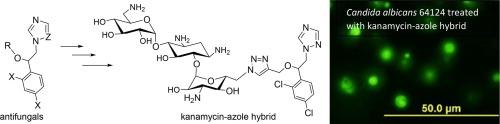
卡那霉素-唑混合物的合成及其抗真菌活性研究
世界卫生组织(WHO)认为,白色念珠菌和新生隐球菌是需要优先治疗的重要真菌病原体。唑类抗真菌剂,包括三唑类、重氮类和噻唑类,被广泛用于治疗真菌感染。鉴于过去通过化学修饰将抗菌卡那霉素转化为抗真菌衍生物所取得的成功,我们合成了一个新的卡那霉素-唑杂交化合物库,并针对一组耐唑和易感的念珠菌和隐球菌菌株进行了测试。结构活性关系(SAR)研究显示,唑环(咪唑与三唑)和苯环上的卤素取代基(F 与 Cl)对抗真菌活性起着关键作用。最值得注意的是,杂交种 13、14 和 15 对具有抗药性的白僵菌、热带僵菌和新变形僵菌菌株具有活性,并且对哺乳动物细胞无毒。使用荧光染料(SYTOXTM)进行的作用模式研究表明,即使在 MIC 为 ¼ 的情况下,真菌活性化合物也能渗透真菌膜。这些发现揭示了新型唑类抗真菌药,可为念珠菌病和隐球菌病提供新的治疗方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


