A new organic–inorganic chloride (H3N–(CH2)6–NH3)[SnCl6]: Crystal structure, thermal analysis, vibrational study, and electrical properties
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
In this work, a new Organic-Inorganic chloride (H3N–(CH2)6-NH3)[SnCl6] (1) has been synthesized and characterized by single crystal X-ray diffraction (XRD), IR, Raman and impedance spectroscopies. The crystallographic study displays that the title compound crystallizes in the triclinic system with the P−1 space group. The vibrational study (IR, Raman) at room temperature confirmed the existence of the organic and inorganic functional groups. Differential scanning calorimetry (DSC) analysis reveals the existence of three reversible phase transitions at T1 = 345/325 K, T2 = 483/443 K, and T3 = 496/465 K (Heating/Cooling). Furthermore, the conductivity analysis and the dielectric properties confirm the presence of these phase transitions. AC-conductivity measurement of (1) has been investigated using complex impedance spectroscopy in frequency and temperature range 10 Hz–5 MHz and 313–523 K, respectively. The study of Nyquist plots showed the contribution of grains and grain boundaries in the electrical study, confirming the existence of a non-Debye type relaxation. The AC conductivity shows that the material has the potential of impedance sensors.
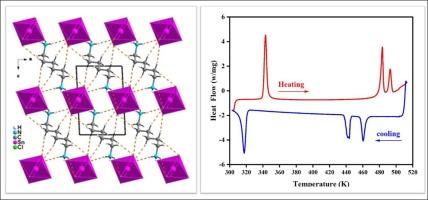
一种新的有机-无机氯化物(H3N-(CH2)6-NH3)[SnCl6]:晶体结构、热分析、振动研究和电学特性
本研究合成了一种新的有机无机氯化物(H3N-(CH2)6-NH3)[SnCl6] (1),并通过单晶 X 射线衍射 (XRD)、红外光谱、拉曼光谱和阻抗光谱对其进行了表征。晶体学研究表明,标题化合物在 P-1 空间群的三菱系中结晶。室温下的振动研究(红外光谱、拉曼光谱)证实了有机和无机官能团的存在。差示扫描量热法(DSC)分析表明,在 T1 = 345/325 K、T2 = 483/443 K 和 T3 = 496/465 K(加热/冷却)存在三个可逆相变。此外,电导率分析和介电特性也证实了这些相变的存在。在频率和温度范围分别为 10 Hz-5 MHz 和 313-523 K 时,使用复阻抗光谱对 (1) 的交流电导率测量进行了研究。奈奎斯特图的研究表明,晶粒和晶界在电学研究中的作用,证实了非戴贝型弛豫的存在。交流电导率表明该材料具有阻抗传感器的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


