Itaconate drives mtRNA-mediated type I interferon production through inhibition of succinate dehydrogenase
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Itaconate is one of the most highly upregulated metabolites in inflammatory macrophages and has been shown to have immunomodulatory properties. Here, we show that itaconate promotes type I interferon production through inhibition of succinate dehydrogenase (SDH). Using pharmacological and genetic approaches, we show that SDH inhibition by endogenous or exogenous itaconate leads to double-stranded mitochondrial RNA (mtRNA) release, which is dependent on the mitochondrial pore formed by VDAC1. In addition, the double-stranded RNA sensors MDA5 and RIG-I are required for IFNβ production in response to SDH inhibition by itaconate. Collectively, our data indicate that inhibition of SDH by itaconate links TCA cycle modulation to type I interferon production through mtRNA release. O’Carroll and Peace et al. provide a mechanism for the induction of type I interferons by the immunomodulatory compound itaconate, which involves inhibition of succinate dehydrogenase and release of mitochondrial double-stranded RNA.

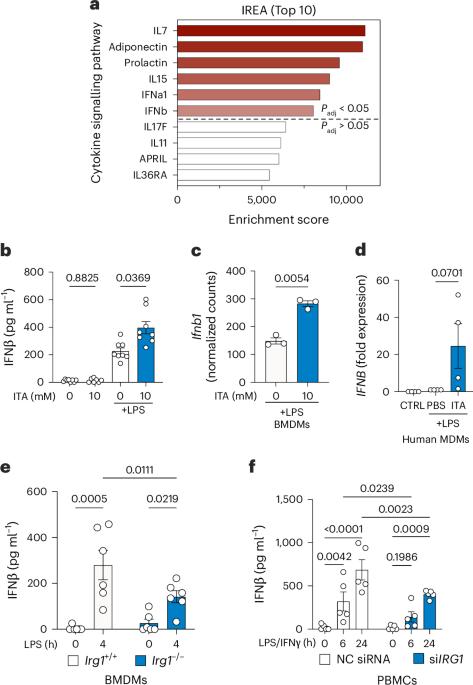
伊塔康酸通过抑制琥珀酸脱氢酶驱动 mtRNA 介导的 I 型干扰素产生
伊塔康酸是炎症巨噬细胞中最高调的代谢物之一,已被证明具有免疫调节特性。在这里,我们发现伊塔康酸可通过抑制琥珀酸脱氢酶(SDH)来促进 I 型干扰素的产生。通过药理学和遗传学方法,我们发现内源性或外源性伊它康酸对 SDH 的抑制会导致双链线粒体 RNA(mtRNA)的释放,而这种释放依赖于 VDAC1 形成的线粒体孔。此外,双链 RNA 传感器 MDA5 和 RIG-I 也是 IFNβ 在伊塔康酸对 SDH 的抑制作用下产生的必要条件。总之,我们的数据表明,伊塔康酸对 SDH 的抑制通过 mtRNA 的释放将 TCA 循环调节与 I 型干扰素的产生联系起来。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


