Omega-3 polyunsaturated fatty acids alleviate hyperuricemic nephropathy by inhibiting renal pyroptosis through GPR120
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Hyperuricemic nephropathy (HN) is characterized by increased serum uric acid levels that incite renal inflammation. While omega-3 polyunsaturated fatty acids (PUFAs) are known for their anti-inflammatory properties, their impact on HN remains unclear. This study explored the effects of omega-3 PUFAs, specifically docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), on HN. Using a mouse model induced by adenine and potassium oxonate, we treated HN mice with DHA, EPA, or both for four weeks. The results showed that omega-3 PUFAs significantly reduced serum uric acid levels and improved kidney function, with DHA, EPA, and their combination showing similar efficacy. Transcriptome sequencing and further analysis revealed that these fatty acids alleviate renal pyroptosis by reducing key markers such as NOD-like receptor pyrin containing 3 (NLRP3), cleaved gasdermin-D, caspase-1, and interleukin-1β. To further investigate the underlying mechanism, we focused on G-protein coupled receptor 120 (GPR120), a receptor activated by DHA. The use of a GPR120 antagonist (AH7614) partially blocked DHA’s effects, while the agonist (TUG891) mimicked its anti-pyroptotic actions. Co-immunoprecipitation assays showed that DHA activates GPR120, leading to its internalization and interaction with β-arrestin2, ultimately inhibiting NLRP3 inflammasome formation and reducing inflammation. Overall, omega-3 PUFAs, particularly through GPR120 activation, appear to protect against renal inflammation in HN by modulating the NLRP3/caspase-1/GSDMD pathway.
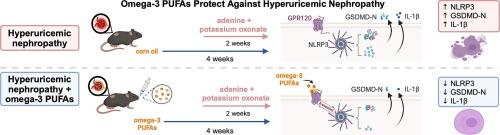
欧米伽-3 多不饱和脂肪酸通过 GPR120 抑制肾脏热蛋白沉积,从而缓解高尿酸血症肾病
高尿酸血症肾病(HN)的特点是血清尿酸水平升高,从而引发肾脏炎症。众所周知,ω-3 多不饱和脂肪酸(PUFA)具有抗炎特性,但它们对高尿酸血症肾病的影响仍不清楚。本研究探讨了欧米伽-3 多不饱和脂肪酸,特别是二十二碳六烯酸(DHA)和二十碳五烯酸(EPA)对 HN 的影响。我们使用腺嘌呤和氧化钾诱导的小鼠模型,用 DHA、EPA 或两者治疗 HN 小鼠四周。结果表明,ω-3 PUFA 能显著降低血清尿酸水平并改善肾功能,其中 DHA、EPA 及其组合具有相似的疗效。转录组测序和进一步分析表明,这些脂肪酸通过减少关键标记物,如含NOD样受体吡咯啉3(NLRP3)、裂解的gasdermin-D、caspase-1和白细胞介素-1β,缓解了肾脏的脓毒症。为了进一步研究其潜在机制,我们重点研究了被 DHA 激活的 G 蛋白偶联受体 120(GPR120)。使用 GPR120 拮抗剂(AH7614)部分阻断了 DHA 的作用,而激动剂(TUG891)则模拟了其抗突眼作用。共免疫沉淀试验表明,DHA 能激活 GPR120,使其内化并与β-arrestin2 相互作用,最终抑制 NLRP3 炎症小体的形成并减轻炎症反应。总之,ω-3 PUFAs,尤其是通过激活 GPR120,似乎可以通过调节 NLRP3/caspase-1/GSDMD通路来保护 HN 肾脏免受炎症侵袭。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


