Application of enaminone ruthenium(II) complexes as catalysts in the transfer hydrogenation of ketones
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
This study reported the synthesis of two new ligands (1,2) and their Ru(II)-enaminone complexes (3,4). The Ru(II) complexes were obtained from the reaction of the [RuCl2(p-cymene)]2 dimers with the new enaminone derivative ligands (1,2). The use of standard spectroscopy techniques entirely characterized the compounds. Single crystal studies investigated the crystal structure of ligand (1) and complexes (3,4). X-ray diffraction data analysis was used to confirm the enaminone form of 1. Also, 3 and 4 exhibited the classical three-legged piano stool structure with Ru(II) coordinated by the nitrogen and oxygen atoms of 1 and a chloride ligand as the legs and the η6-π-bound p-cymene ligand occupies the seat of the piano stool. The Ru(II) complexes have been studied as catalysts in the transfer hydrogenation of acetophenone and other various ketones in the presence of KOtBu. The catalytic conditions were optimized using different substrate/catalyst and base/catalyst ratios. We found that the complexes show good activities and up to 100 % selectivity. The best turnover frequency (1667 h−1) was found for 3 when using acetophenone as the substrate.
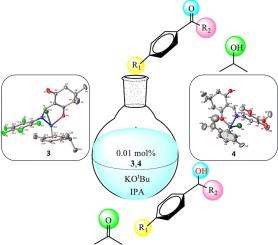
烯酮钌(II)配合物作为催化剂在酮转移加氢反应中的应用
本研究报告了两种新配体(1,2)及其 Ru(II)-烯醌配合物(3,4)的合成。Ru(II)配合物是由[RuCl2(p-cymene)]2二聚体与新的烯胺酮衍生物配体(1,2)反应得到的。利用标准光谱技术对化合物进行了全面表征。单晶研究调查了配体 (1) 和配合物 (3,4) 的晶体结构。此外,3 和 4 呈现出经典的三脚钢琴凳结构,Ru(II) 由 1 的氮原子和氧原子以及氯配体配位,η6-π 结合的对伞花烃配体占据钢琴凳的座位。研究人员将 Ru(II) 复合物作为催化剂,在 KOtBu 存在下进行苯乙酮和其他各种酮的转移加氢反应。使用不同的底物/催化剂和碱/催化剂比例对催化条件进行了优化。我们发现,这些配合物显示出良好的活性和高达 100 % 的选择性。当使用苯乙酮作为底物时,发现 3 的周转频率最高(1667 h-1)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


